1. 70 calories of heat are required to raise the temperature of 2 moles of an ideal gas at constant pressure from 30°C to 35°C. The amount of heat required to raise the temperature of same gas
through the same range (30°C to 35°C) at constant volume (R = 2 cal/mol/K)
a) 30 cal
b) 50 cal
c) 70 cal
d) 90 cal
Explanation:

2. A closed compartment containing gas is moving with some acceleration in horizontal direction. Neglect effect of gravity. Then the pressure in the
compartment is
a) Same everywhere
b) Lower in the front side
c) Lower in the rear side
d) Lower in the upper side
Explanation: A closed compartment containing gas is moving with some acceleration in horizontal direction. Neglect effect of gravity. Then the pressure in the compartment is lower in the front side
3. Three closed vessels A, B and C are at the same temperature T and contain gases which obey the Maxwellian distribution of velocities. Vessel A contains only \[O_{2}\] , B only \[N_{2}\] and C a mixture of
equal quantities of O2 and \[N_{2}\] . If the average speed of the \[O_{2}\] molecules in vessel A is \[V_{1} \] , that of the \[N_{2}\] molecules in vessel B is \[V_{2} \] , the average
speed of the \[O_{2}\] molecules in vessel C is
a) \[\left(V_{1}+V_{2}\right)\diagup 2\]
b) \[V_{1}\]
c) \[\left(V_{1}V_{2}\right)^{1\diagup 2}\]
d) \[\sqrt{3kT / M}\]
Explanation: \[V_{1}\]
4. A box containing N molecules of a perfect gas at temperature \[T_{1}\] and pressure \[P_{1}\] . The number of molecules in the box is doubled keeping the total kinetic energy of the gas same as before. If the
new pressure is \[P_{2}\] and temperature \[T_{2}\] , then
a) \[P_{2}=P_{1},T_{2}=T_{1}\]
b) \[P_{2}=P_{1},T_{2}=\frac{T_{1}}{2}\]
c) \[P_{2}=2P_{1},T_{2}=T_{1}\]
d) \[P_{2}=2P_{1},T_{2}=\frac{T_{1}}{2}\]
Explanation: \[P_{2}=P_{1},T_{2}=\frac{T_{1}}{2}\]
5. A gas in container A is in thermal equilibrium with another gas in container B. both contain equal masses of the two gases in the respective
containers. Which of the following can be true
a) \[P_{A}V_{A}=P_{B}V_{B}\]
b) \[P_{A}=P_{B},V_{A}\neq V_{B}\]
c) \[P_{A}\neq P_{B},V_{A}= V_{B}\]
d) Both b and c
Explanation: Both b and c
6. Two containers of equal volume contain the same gas at pressures \[P_{1}\] and \[P_{2}\] and absolute temperatures \[T_{1}\] and \[T_{2}\] respectively. On joining
the vessels, the gas reaches a common pressure P and common temperature T. The ratio P/T is equal to
a) \[\frac{P_{1}}{T_{1}}+\frac{P_{2}}{T_{2}}\]
b) \[\frac{P_{1}T_{1}+P_{2}T_{2}}{\left(T_{1}+T_{2}\right)^{2}}\]
c) \[\frac{P_{1}T_{2}+P_{2}T_{1}}{\left(T_{1}+T_{2}\right)^{2}}\]
d) \[\frac{P_{1}}{2T_{1}}+\frac{P_{2}}{2T_{2}}\]
Explanation: \[\frac{P_{1}}{2T_{1}}+\frac{P_{2}}{2T_{2}}\]
7. At the top of a mountain a thermometer reads 7°C and a barometer reads 70 cm of Hg. At the bottom of the mountain these read 27°C and 76 cm of Hg
respectively. Comparison of density of air at the top with that of bottom is
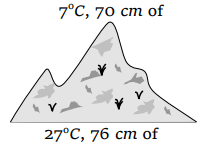
a) 75/76
b) 70/76
c) 76/75
d) 76/70
Explanation: 75/76
8. The root mean square speed of the molecules of a diatomic gas is v. When the temperature is doubled, the molecules dissociate into two atoms.
The new root mean square speed of the atom is
a) \[\sqrt{2v}\]
b) v
c) 2v
d) 4v
Explanation:
9. A vessel is partitioned in two equal halves by a fixed diathermic separator. Two different ideal gases are filled in left (L) and right (R) halves.
The rms speed of the molecules in L part is equal to the mean speed of molecules in the R part. Then the ratio of the mass of a molecule in L part
to that of a molecule in R part is
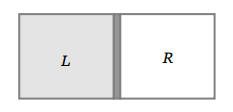
a) \[\sqrt{\frac{3}{2}}\]
b) \[\sqrt{\pi\diagup 4}\]
c) \[\sqrt{2\diagup 3}\]
d) \[3\pi\diagup 8\]
Explanation:

10. A gas is filled in the cylinder shown in the figure. The two pistons are joined by a string. If the gas
is heated, the pistons will
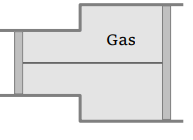
a) Move towards left
b) Move towards right
c) Remain stationary
d) None of these
Explanation: The two pistons are joined by a string. If the gas is heated, the pistons will move towards right