1. A perfect gas at 27°C is heated at constant pressure so as to triple its volume. The temperature of the gas will be
a) 81°C
b) 900°C
c) 627°C
d) 450°C
Explanation:
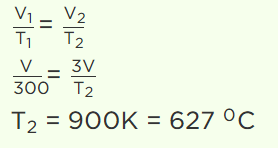
2.The density of a gas at normal pressure and 27°C temperature is 24. Keeping the pressure constant, the density at 127°C will be
a) 6
b) 12
c) 18
d) 24
Explanation:
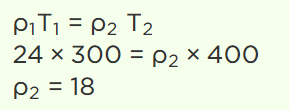
3. The volume of a gas at 20°C is 200 ml. If the temperature is reduced to – 20°C at constant pressure, its volume will be
a) 172.6 ml
b) 17.26 ml
c) 192.7 ml
d) 19.27 ml
Explanation:
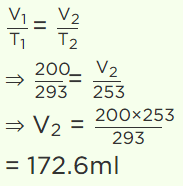
4. Two gases of equal mass are in thermal equilibrium. If \[P_{a},P_{b}\] , and \[V_{a}\] and \[V_{b}\] are their
respective pressures and volumes, then which relation is true
a) \[P_{a}\neq P_{b};V_{a}=V_{b}\]
b) \[P_{a}= P_{b};V_{a}\neq V_{b}\]
c) \[\frac{P_{a}}{V_{a}}= \frac{P_{b}}{V_{b}} \]
d) \[P_{a} V_{a}= P_{b}V_{b}\]
Explanation: \[P_{a} V_{a}= P_{b}V_{b}\]
5. At absolute zero temperature, pressure of a gas will be
a) Zero
b) One atmospheric pressure
c) \[P_{0}\times 273\]
d) \[P_{0}\times 76\]
Explanation: At absolute zero temperature, pressure of a gas will be Zero
6. The gas which obeys Boyle's law for maximum range of temperature is
a) \[CO_{2}\]
b) \[O_{3}\]
c) \[H_{2}\]
d) He
Explanation: The gas which obeys Boyle's law for maximum range of temperature is He
7. The vapour of a substance behaves as a gas
a) Below critical temperature
b) Above critical temperature
c) At 100°C
d) At 1000°C
Explanation: Above critical temperature
8. The temperature below which a gas should be cooled, before it can be liquified by pressure only
is termed as
a) The dew point
b) The freezing point
c) The saturation point
d) The critical point
Explanation: The temperature below which a gas should be cooled, before it can be liquified by pressure only is termed as the critical point
9. It is possible for a substance to coexist in all three phases in equilibrium, when the substance is at
a) Boyle temperature
b) Critical temperature
c) Triple point
d) Dew point
Explanation: It is possible for a substance to coexist in all three phases in equilibrium, when the substance is at triple point
10. When air is filled in the balloon, the pressure and volume both increases while temperature does not change. Here Boyle's law is not obeyed
because
a) Mass of air is negligible
b) Mass of air does not remain constant
c) Air is not perfect gas
d) Pressure inside the balloon is less than that of the atmospheric pressure
Explanation: When air is filled in the balloon, the pressure and volume both increases while temperature does not change. Here Boyle's law is not obeyed because mass of air does not remain constant