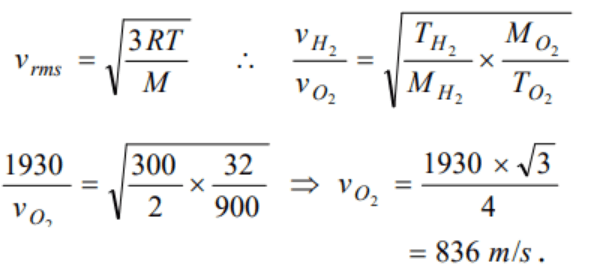1. At a given temperature the r.m.s. velocity of molecules of the gas is
a) Same
b) Proportional to molecular weight
c) Inversely proportional to molecular weight
d) Inversely proportional to square root of
molecular weight
Explanation: At a given temperature the r.m.s. velocity of molecules of the gas is inversely proportional to square root of molecular weight
2. By what factor the r.m.s. velocity will change, if the temperature is raised from 27°C to 327°C
a) \[\sqrt{2}\]
b) 2
c) \[2\sqrt{2}\]
d) 1
Explanation:
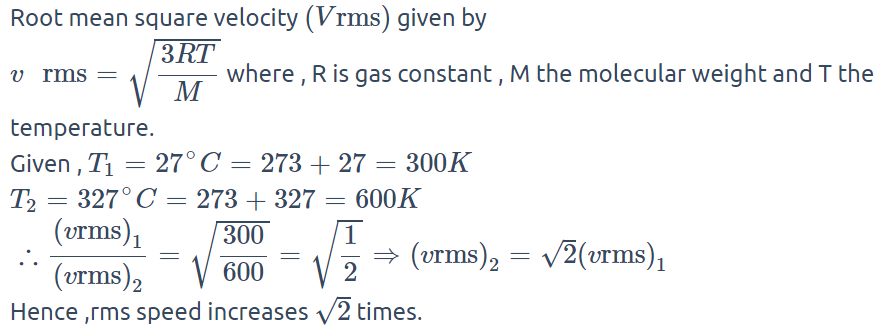
3. The speeds of 5 molecules of a gas (in arbitrary units) are as follows : 2, 3, 4, 5, 6. The root mean
square speed for these molecules is
a) 2.91
b) 3.52
c) 4.00
d) 4.24
Explanation:
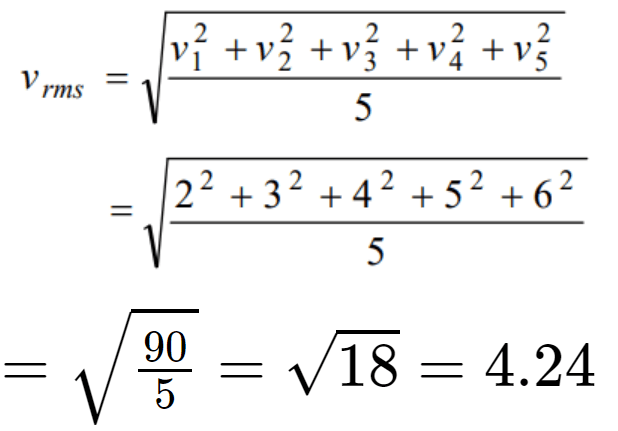
4. At a given temperature the ratio of r.m.s. velocities of hydrogen molecule and helium atom
will be
a) \[\sqrt{2} : 1\]
b) \[ 1: \sqrt{2}\]
c) 1 : 2
d) 2 : 1
Explanation: \[\sqrt{2} : 1\]
5. If the oxygen \[\left(O_{2}\right)\] has root mean square velocity of C \[ms^{-1}\], then root mean square velocity of the
hydrogen \[\left(H_{2}\right)\] will be
a) C \[ms^{-1}\]
b) \[\frac{1}{C} ms^{-1}\]
c) 4C \[ms^{-1}\]
d) \[\frac{C}{4} ms^{-1}\]
Explanation: 4C \[ms^{-1}\]
6. To what temperature should the hydrogen at room temperature (27°C) be heated at constant pressure so that the R.M.S. velocity of its molecules becomes double of its previous value
a) \[1200^{\circ} C\]
b) \[927^{\circ} C\]
c) \[600^{\circ} C\]
d) \[108^{\circ} C\]
Explanation:
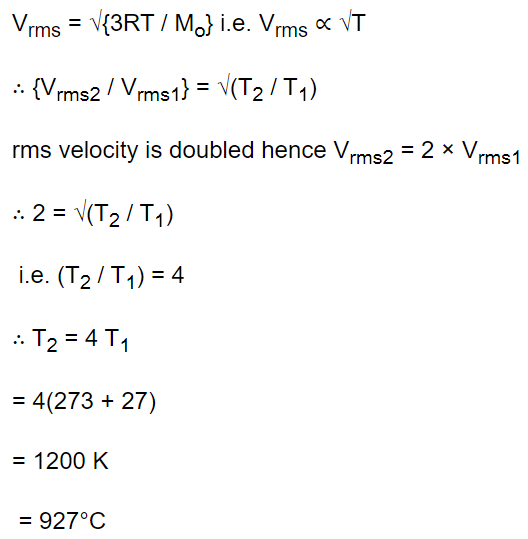
7. The temperature of an ideal gas is reduced from \[927^{\circ} C\] to \[27^{\circ} C\] . The r.m.s. velocity of the molecules
becomes
a) Double the initial value
b) Half of the initial value
c) Four times the initial value
d) Ten times the initial value
Explanation: The temperature of an ideal gas is reduced from \[927^{\circ} C\] to \[27^{\circ} C\] . The r.m.s. velocity of the molecules becomes half of the initial value
8. The r.m.s. speed of the molecules of a gas in a vessel is \[400 ms^{-1}\] . If half of the gas leaks out, at constant temperature, the r.m.s. speed of the
remaining molecules will be
a) \[800 ms^{-1}\]
b) \[400 \sqrt{2}ms^{-1}\]
c) \[400 ms^{-1}\]
d) \[200 ms^{-1}\]
Explanation: \[400 ms^{-1}\]
9. Cooking gas containers are kept in a lorry moving with uniform speed. The temperature of the gas
molecules inside will
a) Increase
b) Decrease
c) Remain same
d) Decrease for some, while increase for others
Explanation: Cooking gas containers are kept in a lorry moving with uniform speed. The temperature of the gas molecules inside will remain same
10. The root mean square speed of hydrogen molecules at 300 K is 1930 m/s. Then the root
mean square speed of oxygen molecules at 900 K will be
a) \[1930 \sqrt{3}m/s\]
b) 836 m / s
c) 643 m / s
d) \[ \frac{1930}{\sqrt{3}}ms^{-1}\]
Explanation: