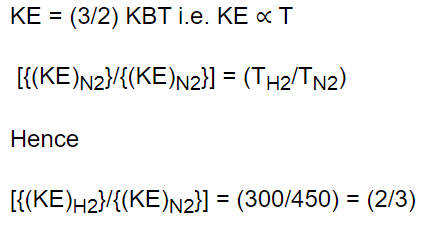1. At a given temperature, the pressure of an ideal gas of density \[\rho\] is proportional to
a) \[\frac{1}{\rho^{2}}\]
b) \[\frac{1}{\rho}\]
c) \[\rho^{2}\]
d) \[\rho\]
Explanation:
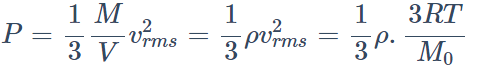
2. Consider a gas with density \[\rho\] and \[\bar{C}\] as the root mean square velocity of its molecules contained in
a volume. If the system moves as whole with velocity v, then the pressure exerted by the gas is
a) \[\frac{1}{3}\rho\bar{C}^{2}\]
b) \[\frac{1}{3}\rho \left(C+V\right)^{2}\]
c) \[\frac{1}{3}\rho \left(C-V\right)^{2}\]
d) \[\frac{1}{3}\rho \left(C^{-2}-V\right)^{2}\]
Explanation: \[\frac{1}{3}\rho\bar{C}^{2}\]
3. If the mean free path of atoms is doubled then the pressure of gas will become
a) P/4
b) P/2
c) P/8
d) P
Explanation: If the mean free path of atoms is doubled then the pressure of gas will become P/2
4. Relationship between P,V, and E for a gas is
a) \[P=\frac{3}{2}EV\]
b) \[V=\frac{2}{3}EP\]
c) \[PV=\frac{3}{2}E\]
d) \[PV=\frac{2}{3}E\]
Explanation: Relationship between P,V, and E for a gas is \[PV=\frac{2}{3}E\]
5. The value of universal gas constant is 8.3 J/mole/K, the mean kinetic energy of 32 gm of
oxygen at – 73°C will be
a) 480 J
b) 4980 J
c) 2490 J
d) The information is incomplete
Explanation:
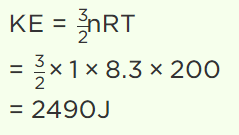
6. For a diatomic gas change in internal energy for unit change in temperature for constant pressure and constant volume is \[U_{1}\] and \[U_{2}\] respectively.
\[U_{1}:U_{2}\] is
a) 5 : 3
b) 3 : 5
c) 1 : 1
d) 5 : 7
Explanation: \[U_{1}:U_{2}\] is 1 : 1
7. If number of molecules of \[H_{2}\] are double than that of \[O_{2}\] , then ratio of kinetic energy of hydrogen
and that of oxygen at 300 K is
a) 1 : 1
b) 1 : 2
c) 2 : 1
d) 1 : 16
Explanation: If number of molecules of \[H_{2}\] are double than that of \[O_{2}\] , then ratio of kinetic energy of hydrogen and that of oxygen at 300 K is 1 : 1
8. The average kinetic energy of a gas at – 23°C and 75 cm pressure is \[5 \times10^{-14}erg\] for \[H_{2}\] . The mean kinetic energy of the \[O_{2}\] at 227°C and 150 cm
pressure will be
a) \[80 \times10^{-14}erg\]
b) \[20 \times10^{-14}erg\]
c) \[40 \times10^{-14}erg\]
d) \[10 \times10^{-14}erg\]
Explanation:

9. The ratio of mean kinetic energy of hydrogen and oxygen at a given temperature is
a) 1 : 16
b) 1 : 8
c) 1 : 4
d) 1 : 1
Explanation: The ratio of mean kinetic energy of hydrogen and oxygen at a given temperature is 1 : 1
10. The ratio of mean kinetic energy of hydrogen and nitrogen at temperature 300 K and 450 K
respectively is
a) 3 : 2
b) 2 : 3
c) 2 : 21
d) 4 : 9
Explanation: