1. Saturated vapour is compressed to half its volume without any change in temperature, then the pressure will be
a) Doubled
b) Halved
c) The same
d) Zero
Explanation: Saturated vapour is compressed to half its volume without any change in temperature, then the pressure will be the same
2. Air is pumped into an automobile tube upto a pressure of 200 kPa in the morning when the air temperature is 22°C. During the day, temperature rises to 42°C and the tube expands by 2%. The
pressure of the air in the tube at this temperature, will be approximately
a) 212 kPa
b) 209 kPa
c) 206 kPa
d) 200 kPa
Explanation:

3. At what temperature volume of an ideal gas at 0°C becomes triple
a) 546°C
b) 182°C
c) 819°C
d) 646°C
Explanation: 546°C
4. If an ideal gas has volume V at 27°C and it is heated at a constant pressure so that its volume becomes 1.5V. Then the value of final temperature
will be
a) 600°C
b) 177°C
c) 817°C
d) None of these
Explanation: 177°C
5. The temperature of an ideal gas at atmospheric pressure is 300K and volume 1m3. If temperature and volume become double, then pressure will be
a) \[10^{5} N\diagup m^{2}\]
b) \[2\times10^{5} N\diagup m^{2}\]
c) \[0.5\times10^{5} N\diagup m^{2}\]
d) \[4\times10^{5} N\diagup m^{2}\]
Explanation:
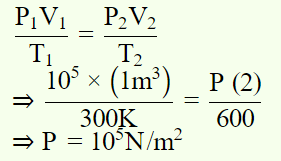
6. What is the mass of 2 litres of nitrogen at 22.4 atmospheric pressure and 273K
a) 28g
b) \[14\times22.4 g \]
c) 56 g
d) None of these
Explanation: 28g
7. The value of PV/T for one mole of an ideal gas is nearly equal to
a) 2 \[J mol^{-1}k^{-1} \]
b) 8.3 cal \[mol^{-1}k^{-1} \]
c) 4.2 \[J mol^{-1}k^{-1} \]
d) 2 cal \[mol^{-1}k^{-1} \]
Explanation: The value of PV/T for one mole of an ideal gas is nearly equal to 2 cal \[mol^{-1}k^{-1} \]
8. A tyre kept outside in sunlight bursts off after sometime because of
a) Increase in pressure
b) Increases in volume
c) Both (a) and (b)
d) None of these
Explanation: A tyre kept outside in sunlight bursts off after sometime because of increase in pressure
9. If the volume of the gas containing n number of molecules is V, then the pressure will decrease due to force of intermolecular attraction in the
proportion
a) n / v
b) \[n / V^{2} \]
c) \[\left(n / V\right)^{2} \]
d) \[1 / V^{2} \]
Explanation: \[\left(n / V\right)^{2} \]
10. In Boyle's law what remains constant
a) PV
b) TV
c) \[\frac{V}{T}\]
d) \[\frac{P}{T}\]
Explanation: In Boyle's law, PV remains constant