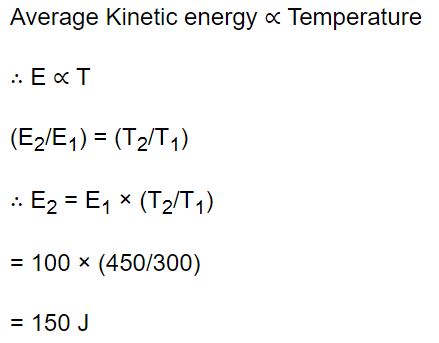1. According to the kinetic theory of gases, total energy of a gas is equal to
a) Potential energy
b) Kinetic energy
c) Both (a) and (b)
d) None of the above
Explanation: According to the kinetic theory of gases, total energy of a gas is equal to kinetic energy
2. The average kinetic energy of a gas molecule can be determined by knowing
a) The number of molecules in the gas
b) The pressure of the gas only
c) The temperature of the gas only
d) None of the above is enough by itself
Explanation: The average kinetic energy of a gas molecule can be determined by knowing the temperature of the gas only
3. Mean kinetic energy (or average energy) per gm molecule of a monoatomic gas is given by
a) \[\frac{3}{2}RT\]
b) \[\frac{1}{2}KT\]
c) \[\frac{1}{2}RT\]
d) \[\frac{3}{2}KT\]
Explanation: Mean kinetic energy (or average energy) per gm molecule of a monoatomic gas is given by \[\frac{3}{2}RT\]
4. A sealed container with negligible coefficient of volumetric expansion contains helium (a monoatomic gas). When it is heated from 300 K to
600 K, the average K.E. of helium atoms is
a) Halved
b) Unchanged
c) Doubled
d) Increased by factor \[\sqrt{2}\]
Explanation: The average K.E. of helium atoms is doubled
5. The time average of the kinetic energy of one molecule of a gas taken over a long period of time
a) Is proportional to the square root of the absolute temperature of the gas
b) Is proportional to the absolute temperature of the gas
c) Is proportional to the square of the absolute temperature of the gas
d) Does not depend upon the absolute temperature of the gas
Explanation: The time average of the kinetic energy of one molecule of a gas taken over a long period of time is proportional to the absolute temperature of the gas
6. The kinetic energy per gm mol for a diatomic gas at room temperature is
a) 3 RT
b) \[\frac{5}{2}RT\]
c) \[\frac{3}{2}RT\]
d) \[\frac{1}{2}RT\]
Explanation: The kinetic energy per gm mol for a diatomic gas at room temperature is \[\frac{5}{2}RT\]
7. At which of the following temperature would the molecules of a gas have twice the average kinetic
energy they have at 20°C
a) 40°C
b) 80°C
c) 313°C
d) 586°C
Explanation:
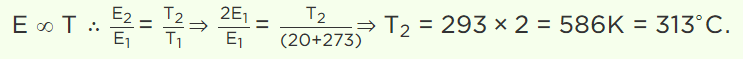
8. The kinetic energy of translation of 20 gm of oxygen at 47°C is (molecular wt. of oxygen is 32 gm/mol and R = 8.3 J/mol/K)
a) 2490 joules
b) 2490 ergs
c) 830 joules
d) 124.5 joules
Explanation:
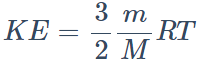
9. The kinetic energy of one gram molecule of a gas at normal temperature and pressure
is (R = 8.31 J /mole - K)
a) \[0.56\times 10^{4} J\]
b) \[1.3\times 10^{2} J\]
c) \[2.7\times 10^{2} J\]
d) \[3.4\times 10^{3} J\]
Explanation:
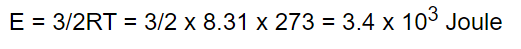
10. The mean kinetic energy of a gas at 300 K is 100 J. The mean energy of the gas at 450 K is equal to
a) 100 J
b) 3000 J
c) 450 J
d) 150 J
Explanation: