1. Sodium metal crystallises in bcc lattice with the cell edge, a =
4.29 Å. What is the radius of sodium atom?
a) 1.79 Å
b) 1.89Å
c) 4 Å
d) 3.2 Å
Explanation:

2. Which of the following statements is wrong ?
a) The coordination number of each type of ion in CsCl
crystal is 8.
b) A metal that crystallizes in bcc structure has a
coordination number of 12.
c)A unit cell of an ionic crystal shares some of its ions with
other unit cells
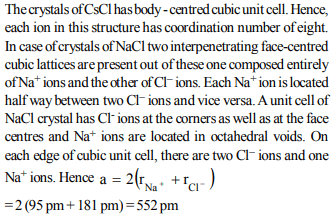
d) The length of the unit cell in NaCl is 552 pm (rNa+=95 pm ; rCl-=181 pm)
Explanation:
3.Argon crystallizes in a structure in which the atoms are
located at the postions (0,0,0)
\[\left(0,\frac{1}{2},\frac{1}{2}\right),\left(\frac{1}{2}, 0, \frac{1}{2}\right) and\left( \frac{1}{2},\frac{1}{2},0\right)\]
The unit cell is
a) simple cubic
b) body-centred cubic
c) face-centred cubic
d) hexagonal close packed
Explanation: The planes indicate, the unit cell is fcc
4. In NaCl, the centre-to-centre nearest-neighbour distance of
ions is
a) \[\frac{1}{4}a\]
b) \[\frac{\sqrt{3}}{2}a\]
c) \[\frac{1}{2}a\sqrt{2}\]
d) \[\frac{1}{2}a\]
Explanation:
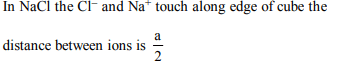
5.Consider the radii 0.095 nm \[\left(Na^{+}\right)\] , 0.181 nm \[\left(Cl^{-}\right)\] ,
0.074 nm \[\left(Zn^{2+}\right)\] , 0.184 nm \[\left(S^{2-}\right)\] , 0.068 nm \[\left(Ti^{4+}\right)\] ,
0.140 nm \[\left(O^{2-}\right)\] , 0.169 nm \[\left(Cs^{+}\right)\] . Choose the correct option
from among the following. (Use radius ratio rules)
a) \[Na^{+}\] ions are packed in octahedral holes between the
planes of close-packed \[Cl^{-}\] ions.
b) \[Zn^{2+}\] ions are packed in tetrahedral holes
c) \[CS^{+}\] ions are packed in a simple cubic array of
\[Cl^{-}\] ions
d) All of these
Explanation:

6. Which of the following is the incorrect statement
a) NaCl has 6 : 6 coordination and CsCl has 8 : 8 coordination.
b) In \[Na_{2}O\] each oxide ion is coordinated by \[8Na^{+}\] ions
and each \[Na^{+}\] ion by 4 oxide ions
c) NaCl structure transform to CsCl structure on heating
d) In \[CaF_{2}\] structure each \[F^{-}\] ion is coordinated by 4
\[Ca^{2+}\] and vice versa.
Explanation: CsCl (8 : 8 coordination) transform to NaCl (6 : 6 coordination) on heating but reverse is not possible.
7. The compound having the lowest lattice energy
a) NaF
b) CsF
c) KF
d) RbF
Explanation: The coulombic attractions between Cs+ and F- ions is lowest due to larger size of Cs+.
8. In the unit cell of KCl, \[Cl^{-}\] ions constitute ccp and \[K^{+}\] ions
fall into the octahedral holes. These holes are
a) one at the centre and 12 are at the centres of the edges
b) one at the centre and 6 at the centres of the faces
c) 8 at the corners of 8 small cubes forming the unit cell
d) None is correct
Explanation: One octahedral void is present at the body centre of the cube and 12 octahedral voids are present on the centres of the cube.
9. The non stoichiometric compound \[Fe_{0.94}O\] is formed when
x % of \[Fe^{2+}\] ions are replaced by as many \[\frac{2}{3}Fe^{3+}\] ions, x is
a) 18
b) 12
c) 15
d) 6
Explanation:
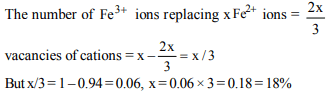
10. The incorrect statement for the sulphur atom of ZnS is
a) As \[S^{2-}\] is larger than \[Zn^{2+}\] only 4 rather than 6 or 8
\[S^{2-}\] can be packed around \[Zn^{2+}\]
b) Its structure is similar to diamond except that alternate
atoms are Zn and S
c) As \[S^{2-}\] is larger than \[Zn^{2+}\] only 6 rather than 8 or 4
sulphide ions can be placed around \[Zn^{2+}\] ions
d) ZnS is a covalent compound
Explanation: Statement (a) is correct.