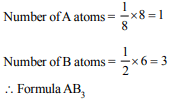1. For two ionic solids CaO and KI, identify the wrong
statement amongst the following :
a) The lattice energy of CaO is much large than that of KI
b) KI is more soluble in water
c) KI has higher melting point
d) CaO has higher melting point
Explanation: KI has higher melting point
2. For a covalent solid, the units which occupy lattice points
are:
a) atoms
b) ions
c) molecules
d) electrons
Explanation: atoms
3. Graphite is a
a) molecular solid
b) covalent solid
c) ionic solid
d) metallic solid
Explanation: Graphite is a covalent solid
4. Which of the following is not a crystalline solid?
a) KCl
b) CsCl
c) Glass
d) Rhombic S
Explanation: Glass is amorphous solid
5. Among solids, the highest melting point is exhibited by
a) Covalent solids
b) Ionic solids
c) Pseudo solids
d) Molecular solids
Explanation: Covalent as in case of diamond
6. The major binding force of diamond, silicon and quartz is
a) electrostatic force
b) electrical attraction
c) covalent bond force
d)non-covalent bond force
Explanation: Covalent bond force
7. The maximum proportion of available volume that can be
filled by hard spheres in diamond is
a) 0.52
b) 0.34
c) 0.32
d) 0.68
Explanation: The volume to be filled by hard spheres in diamond is 0.34.
8. The packing fraction for a body-centred cubic is
a) 0.42
b) 0.53
c) 0.68
d) 0.82
Explanation: The p.f. for body centred cube = 0.68
9.In a solid AB having the NaCl type structure, ‘A’ atoms
occupy the corners of the cubic unit cell. If all the facecentred
atoms along one of the axes are removed, then the
resultant stoichiometry of the solid is
a) \[AB_{2}\]
b) \[A_{2}B\]
c) \[A_{4}B_{3}\]
d) \[A_{3}B_{4}\]
Explanation:

10. A substance \[A_{x}B_{y}\] crystallizes in a face centred cubic (fcc)
lattice in which atoms ‘A’ occupy each corner of the cube
and atoms ‘B’ occupy the centres of each face of the cube.
Identify the correct composition of the substance \[A_{x}B_{y}\]
a) \[AB_{3}\]
b) \[A_{4}B_{3}\]
c) \[A_{3}B\]
d) Composition can’t be specified
Explanation: