1. The edge length of a face centered cubic cell of an ionic
substance is 508 pm. If the radius of the cation is 110 pm, the
radius of the anion is
a) 288 pm
b)398 pm
c) 618 pm
d) 144 pm
Explanation:
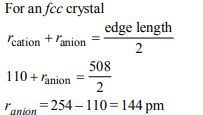
2. Percentages of free space in cubic close packed structure
and in body centered packed structure are respectively
a) 30% and 26%
b) 26% and 32%
c) 32% and 48%
d) 48% and 26%
Explanation: Packing fraction is defined as the ratio of the volume of the unit cell that is occupied by the spheres to the total volume of the unit cell. P.F. for ccp and bcc are 0.74 and 0.68 respectively. So, the free space in ccp and bcc are 26% & 32% respectively
3. Lithium forms body centred cubic structure. The length of
the side of its unit cell is 351 pm. Atomic radius of the lithium
will be :
a) 75 pm
b) 300 pm
c) 240 pm
d) 152 pm
Explanation:
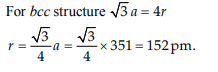
4. Which of the following exists as covalent crystals in the solid
state ?
a) Iodine
b) Silicon
c) Sulphur
d) Phosphorus
Explanation: Among the given crystals, only silicon exists as a covalent solid. It has diamond like structure.
5. The radius of \[Li^{+}\] ion is 60pm and that of \[F^{-}\] is 136 pm.
Structure of LiF and coordination number is
a) Like NaCl, C.No. = 6
b) Like CsCl, C.No. = 8
c) Anti fluoride, C.No. = 8
d) None of these
Explanation:
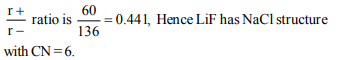
6. Atoms A are arranged in ccp array. Atoms B occupy all
octahedral voids and half of the tetrahedral voids. The formula
of compound AB is
a) \[AB_{2}\]
b) \[A_{2}B\]
c) AB
d) \[A_{2}B_{3}\]
Explanation: \[AB_{2}\]
7. If Germanium crystallises in the same way as diamond, then
which of the following statement is not correct?
a) Every atom in the structure is tetrahedrally bonded to 4
atoms.
b) Unit cell consists of 8 Ge atoms and co-ordination number
is 4.
c) All the octahedral voids are occupied.
d) All the octahedral voids and 50% tetrahedral voids remain
unoccupied
Explanation: All the octahedral voids are occupied
8. Amorphous solid may be classified as
a) Isotropic and superheated solid
b) Isoenthalpic and superheated liquid
c) Isotropic and supercooled liquids
d) Anisotropic and supercooled liquids
Explanation: Amorphous solids are isotropic and supercooled liquids
9. In a solid lattice the cation has left a lattice site and is located
at an interstitial position, the lattice defect is :
a) Interstitial defect
b) Valency defect
c) Frenkel defect
d) Schottky defect
Explanation: Frenkel defect is due to dislocation of ion from its usual lattice site to interstitial position.
10. Which of the following will not adopt CsCl structure?
a) CsF
b) CsBr
c) CsS
d) CsCN
Explanation: CsF will not adopt CsCl structure due to small size of F-