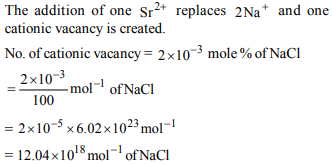1. In KBr crystal structure, the second nearest neighbour of \[K^{+}\] ion will be.............. and its number is
a) \[K^{+},12\]
b) \[K^{+},6\]
c) \[Br^{-},12\]
d) \[Br^{-},6\]
Explanation:

2.Which of the following expression is correct for CsCl unit
cell with lattice parameter a
a) \[r_{Cs^{+}}+r_{Cl^{-}}=\frac{3a}{2}\]
b) \[r_{Cs^{+}}+r_{Cl^{-}}=\frac{\sqrt{3}a}{2}\]
c) \[r_{Cs^{+}}+r_{Cl^{-}}=\frac{a}{\sqrt{2}}\]
d) \[r_{Cs^{+}}+r_{Cl^{-}}=2a\]
Explanation: CsCl has a bcc structure ions touching along body diagonal
3. For a cubic crystal, the lattice parameter, a is 300 pm. The
spacing (d) for (111) plane will be
a) 212.1 pm
b) 259.8 pm
c) 173.2 pm
d) 300 pm
Explanation:
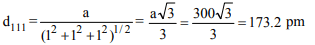
4. Which of the following is a correct statement ?
a) Bonding in metallic crystals is directional
b) Diamond has two dimensional crystal lattice
c) Co-ordination number of bcc lattice is 12
d) A ccp structure has 8 tetrahedral and 4 Octahedral voids
Explanation: The statement (d) is correct.
5. \[M_{2}X\] has anti fluorite structure. In such structure
a) \[X^{--}\] ions occupy all the 8 octahedral voids
b) each \[X^{--}\] is surrounded by \[4M^{+}\] in tetrahedral
arrangement
c) larger cations occupy the position of \[F^{-}\] ions and smaller
anions that of \[C^{++}\] ions
d) smaller cations occupy the position of \[F^{-}\] ions and larger
anions that of \[C^{++}\] ions
Explanation: The statement (d) is correct.
6. The coordination number X (........) of each ion in KBr is
changed to Y (..........) by.............
a) X = 6 to Y = 8 applying higher temperature
b) X = 8 to Y = 6 applying high pressure
c) X = 6 to Y = 8 applying high pressure
d) None of these
Explanation: The increase in pressure results in decrease in size of ions (more in case of anion than cation), the r+/ r- increases and the coordination number also increase.
7. The unit cell of diamond is made up of
a) 6 C atoms, 4 atoms constitute ccp and 2 atoms occupy
the half of octahedral voids
b) 12 C atoms, 4 atoms form fcc lattice and 8 atoms occupy
all tetrahedral holes,
c) 8 C atoms, 4 atoms constitute ccp and 4 atoms occupy
all the octahedral voids
d) 8 C atoms, 4 atoms form fcc lattice and 4 atoms occupy
half of the tetrahedral voids alternately
Explanation: The statement (d) is correct
8. CaO and NaCl have the same crystal structure and nearly the
same ionic radii. If x is the lattice energy of NaCl, the lattice
energy of CaO is very nearly
a) X
b) 2X
c) \[\frac{X}{2}\]
d) 4X
Explanation:

9. Doping of AgCl crystals with \[CdCl_{2}\] results in
a) Frenkel defect
b) Schottky defect
c) Substitutional cation vacancy
d) Formation of F - centres
Explanation: The statement (c) is correct.
10. NaCl is doped with \[2\times 10^{-3} mole\] % of SrCl2 . The
concentration of cation vacancies is
a) \[12.04\times 10^{20}\] per mol
b) \[3.01\times 10^{18}\] per mol
c) \[6.02\times 10^{18}\] per mol
d) \[12.04\times 10^{18}\] per mol
Explanation: