1. The number of unit cells in 58.8 g of NaCl is nearly
a) \[6 × 10^{20}\]
b) \[3 × 10^{22}\]
c) \[1.5 × 10^{23}\]
d) \[0.5 × 10^{24}\]
Explanation:
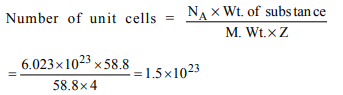
2. An element has bcc structure having unit cells \[12.08 × 10^{23}\] .
The number of atoms in these cells is
a) \[12.08 × 10^{23}\]
b) \[24.16 × 10^{23}\]
c) \[48.38 × 10^{23}\]
d) \[12.08 × 10^{22}\]
Explanation:
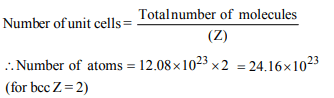
3. An element (atomic mass = 100 g / mol) having bcc structure
has unit cell edge 400 pm. Then, density of the element is
a) 10.376 \[g/cm^{3}\]
b) 5.188 \[g/cm^{3}\]
c) 7.289 \[g/cm^{3}\]
d) 2.144 \[g/cm^{3}\]
Explanation:
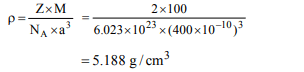
4.The edge length of face centred unit cubic cell is 508 pm. If
the radius of the cation is 110 pm, the radius of one anion is
a) 144 pm
b) 288 pm
c) 618 pm
d) 398 pm
Explanation:

5. When electrons are trapped into the crystal in anion vacancy,
the defect is known as :
a) Schottky defect
b) Frenkel defect
c) Stoichiometric defect
d) F-centres
Explanation: F-centres
6. In the laboratory, sodium chloride is made by burning sodium
in the atmosphere of chlorine. The salt obtained is yellow in
colour. The cause of yellow colour is
a) Presence of \[Na^{+}\] ions in the crystal lattice
b) Presence of \[Cl^{-}\] ions in the crystal lattice
c) Presence of electrons in the crystal lattice
d) Presence of face centred cubic crystal lattice
Explanation: Due to presence of F-centres
7. Schottky defect in crystals is observed when
a) unequal number of cations and anions are missing from
the lattice
b)equal number of cations and anions are missing from the
lattice
c) an ion leaves its normal site and occupies an interstitial
site
d) density of the crystal is increased
Explanation: It is stoichiometric defect and it is observed when equal number of cations and anions are missing from the lattice site.
8. Which of the following has Frenkel defects?
a) Sodium chloride
b) Graphite
c) Silver bromide
d) Diamond
Explanation: AgBr exhibit Frenkel defect
9. Which of the following crystals does not exhibit Frenkel
defect?
a) AgBr
b) AgCl
c) KBr
d) ZnS
Explanation: KBr does not exhibit Frenkel defect.
10. Due to Frenkel defect, the density of ionic solids
a) decreases
b) increases
c) neither (a) nor (b)
d) does not change
Explanation: No change in density