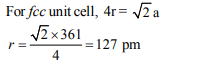1.A metal crystallizes with a face-centered cubic lattice. The
edge length of the unit cell is 408 pm. The diameter of the
metal atom is :
a) 288 pm
b) 408 pm
c) 144 pm
d) 204 pm
Explanation:
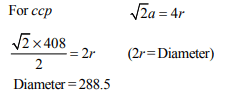
2. A metal has a fcc lattice. The edge length of the unit cell is 404
pm. The density of the metal is 2.72 g cm-3. The molar mass of
the metal is : \[(N_{a}\] Avogadro’s constant= \[6.02 × 10^{23}mol ^{-1})\]
a) 30 g \[mol ^{-1}\]
b) 27 g \[mol ^{-1}\]
c) 20 g \[mol ^{-1}\]
d) 40 g \[mol ^{-1}\]
Explanation:

3. Which of the following statements about the interstitial
compounds is incorrect ?
a) They are chemically reactive
b)They are much harder then the pure metal
c) They have higher melting points than the pure metal
d) They retain metallic conductivity
Explanation: In interstitial compounds small atoms like H, B & C enter into the void sites between the packed atoms of crystalline metal. They retain metallic conductivity and are chemically inert.
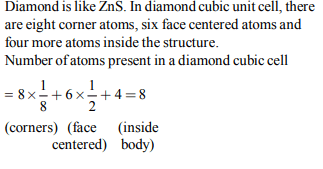
4. The number of carbon atoms per unit cell of diamond unit cell
is :
a) 8
b) 6
c) 1
d) 4
Explanation:
5. Na and Mg crystallize in bcc and fcc type crystals respectively,
then the number of atoms of Na and Mg present in the unit
cell of their respective crystal is
a) 4 and 2
b) 9 and 14
c) 14 and 9
d) 2 and 4
Explanation:
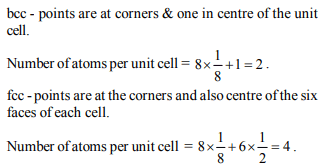
6. How many unit cells are present in a cube-shaped ideal
crystal of NaCl of mass 1.00 g ?
[Atomic masses \[Na=23, Cl=35.5]\]
a) \[5.14 × 10^{21}\] unit cells
b) \[1.28 × 10^{21}\] unit cells
c) \[1.71 × 10^{21}\] unit cells
d) \[2.57 × 10^{21}\] unit cells
Explanation:
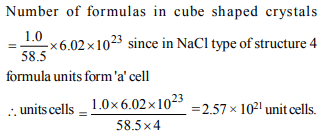
7. An ionic compound has a unit cell consisting of A ions at the
corners of a cube and B ions on the centres of the faces of
the cube. The empirical formula for this compound would be
a) \[A_{3}B\]
b) \[AB_{3}\]
c) \[A_{2}B\]
d) AB
Explanation:

8. Total volume of atoms present in a face-centred cubic unit
cell of a metal is (r is atomic radius)
a) \[\frac{12}{3}\pi r^{3}\]
b) \[\frac{16}{3}\pi r^{3}\]
c) \[\frac{20}{3}\pi r^{3}\]
d) \[\frac{24}{3}\pi r^{3}\]
Explanation:
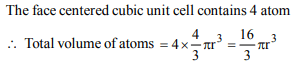
9.In a compound, atoms of element Y form ccp lattice and those
of element X occupy \[2/3^{rd}\] of tetrahedral voids. The formula
of the compound will be
a) \[X_{4}Y_{3}\]
b) \[X_{2}Y_{3}\]
c) \[X_{2}Y\]
d) \[X_{3}Y_{4}\]
Explanation:

10. Copper crystallises in fcc with a unit cell length of 361 pm.
What is the radius of copper atom?
a) 127 pm
b) 157 pm
c) 181 pm
d) 108 pm
Explanation: