1. The uncertainty in the position of an electron \[\left(mass=9.1 × 10^{-28}g\right)\] moving with a velocity of \[3.0 × 10^{4}cms^{-1}\] accurate upto 0.011% will be
a) 1.92 cm
b) 7.68 cm
c) 0.175 cm
d) 3.84 cm.
Explanation:
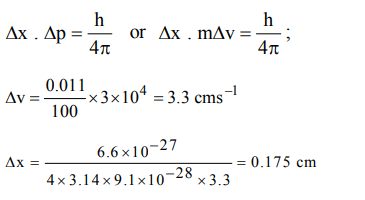
2. The uncertainty in the momentum of an electron is \[1.0 × 10^{-5}kg ms^{-1}\] . The uncertainty in its position will be \[\left(h = 6.62 × 10^{-34}kg m^{2}s^{-1}\right)\]
a) \[1.05 × 10^{-26}m \]
b) \[1.05 × 10^{-28}m \]
c) \[5.27 × 10^{-30}m \]
d) \[5.25 × 10^{-28}m \]
Explanation:
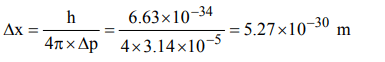
3. Azimuthal quantum number determines the
a) size
b) spin
c) orientation
d) angular momentum of orbitals
Explanation:
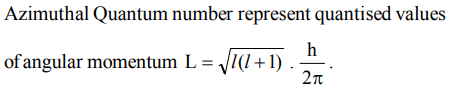
4. When the value of azimuthal quantum number, l = 2, value of ‘n’ will be
a) 3
b) 4
c) 5
d) any one of these
Explanation: For l = 2 (d subshell) n \[\geq\] 3
5. The values of quantum numbers n, l and m for the 5th electron of Boron will be
a) n = 1, l = 0, m = –1
b) n = 2, l = 1, m = –1
c) n = 2, l = 2, m = –1
d) n = 1, l = 2, m = –1
Explanation:
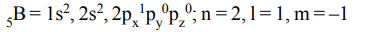
6. The total number of orbitals in a shell with principal quantum
number ‘n’ is
a) 2n
b) \[2n^{2}\]
c) \[n^{2}\]
d) n
Explanation: Number of orbitals with principal quantum number n = n2
7. Which one of the following set of quantum numbers is not possible for 4p electron?
a) \[n = 4, l = 1, m = –1,m_{s}=+\frac{1}{2}\]
b) \[n = 4, l = 1, m = 0,m_{s}=+\frac{1}{2}\]
c) \[n = 4, l = 1, m = 2,m_{s}=+\frac{1}{2}\]
d) \[n = 4, l = 1, m = –1,m_{s}=-\frac{1}{2}\]
Explanation: For 4p electron n = 4, l = 1, m = –1, 0 + 1 and s = +½ or –½
8. The maximum number of electrons in subshell with l = 2 and n = 3 is
a) 2
b) 6
c) 12
d) 10
Explanation: l = 2 for d subshell hence 10 electrons
9. An electron has principal quantum number 3. The number of its (i) sub-shells and (ii) orbitals would be respectively
a) 3 and 5
b) 3 and 7
c) 3 and 9
d) 2 and 5
Explanation: When n = 3 we have s,p and d sub-shells. Hence subshells = 3 and obitals 1 + 3 + 5 = 9
10. An \[\overline{e}\] has magnetic quantum number as –3, what is its principal quantum number
a) 1
b) 2
c) 3
d) 4
Explanation: When m = – 3, l = 3
n = 4