1. The energy absorbed by each molecule \[\left(A_{2}\right)\] of a substance is \[4.4×10^{-19}j \] and bond energy per molecule is \[4.0×10^{-19}j \] . The kinetic energy of the molecule per atom will be:
a) \[2.2×10^{-19}J\]
b) \[2.0×10^{-19}J\]
c) \[4.0×10^{-20}J\]
d) \[2.0×10^{-20}J\]
Explanation:
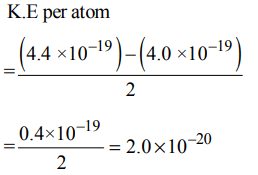
2. Maximum number of electrons in a subshell of an atom is determined by the following
a) \[2\ell+1\]
b) \[4\ell-2\]
c) \[2n^{2}\]
d) \[4\ell+2\]
Explanation: The number of sub shell is (2 l + 1). The maximum number of electrons in the sub shell is 2 (2 l + 1) = (4 l + 2)
3. Which of the following is not permissible arrangement of electrons in an atom?
a) n = 5, l = 3, m = 0, s = + 1/2
b) n = 3, l = 2, m = – 3, s = – 1/2
c) n = 3, l = 2, m = – 2, s = – 1/2
d) n = 4, l = 0, m = 0, s = – 1/2
Explanation: m = – l to +l, through zero thus for l = 2, values of m will be – 2, –1, 0, + 1, + 2
Therefore for l = 2, m cannot have the value –3.
4. A 0.66 kg ball is moving with a speed of 100 m/s. The associated wavelength will be \[\left(h=6.6*10^{-34} Js\right)\] :
a) \[1.0 * 10^{-32}m\]
b) \[6.6 * 10^{-32}m\]
c) \[6.6 * 10^{-34}m\]
d) \[1.0 * 10^{-35}m\]
Explanation:
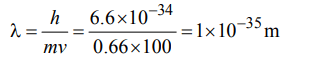
5. The total number of atomic orbitals in fourth energy level of an atom is :
a) 8
b) 16
c) 32
d) 4
Explanation: Total no. of atomic orbitals in a shell = n2
Given n = 4; Hence number of atomic orbitals in 4th shell will be 16.
6. The energies \[E_{1} and E_{2}\] of two radiations are 25 eV and 50 eV, respectively. The relation between their wavelengths i.e., \[\lambda_{1} \] and \[\lambda_{2} \] will be :
a) \[\lambda_{1}=\lambda_{2}\]
b) \[\lambda_{1}=2\lambda_{2}\]
c) \[\lambda_{1}=4\lambda_{2}\]
d) \[\lambda_{1}=\frac{1}{2}\lambda_{2}\]
Explanation:
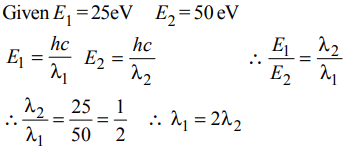
7. If n = 6, the correct sequence for filling of electrons will be :
a) \[ns\rightarrow\left(n-2\right)f\rightarrow\left(n-1\right)d\rightarrow np\]
b) \[ns\rightarrow\left(n-1\right)f\rightarrow\left(n-2\right)d\rightarrow np\]
c) \[ns\rightarrow\left(n-2\right)f\rightarrow np\rightarrow\left(n-1\right)d \]
d) \[ns\rightarrow np\rightarrow \left(n-1\right)d\rightarrow\left(n-2\right)f\]
Explanation:
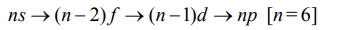
8. According to the Bohr Theory, which of the following transitions in the hydrogen atom will give rise to the least energetic photon ?
a) n = 6 to n = 1
b) n = 5 to n = 4
c) n = 6 to n = 5
d) n = 5 to n = 3
Explanation:
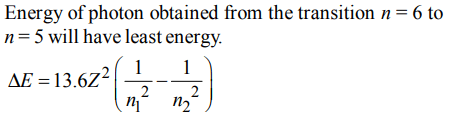
9. The value of Planck’s constant is \[6.63 × 10^{-34} Js\] . The speed of light is \[3 × 10^{17}\] nm \[s^{-1}\] Which value is closest to the wavelength in nanometer of a quantum of light with frequency of \[6 × 10^{15} s^{-1}\] ?
a) 25
b) 50
c) 75
d) 10
Explanation:
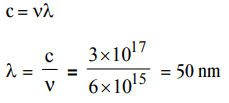
10. What is the maximum numbers of electrons that can be associated with the following set of quantum numbers? n = 3, l = 1 and m = –1
a) 6
b) 4
c) 2
d) 10
Explanation: n = 3 for 3rd shell
l = 1 for p sub shell
m = – 1 is possible for two electrons present in an orbital.