1. The number of electrons and neutrons of an element is 18 and 20 respectively. Its mass number is
a) 2
b) 17
c) 37
d) 38
Discussion
Explanation: For neutral atom . No. of p = No. of e- = 18 and A = Z + No. of neutrons = 18 + 20 = 38
2. The hydride ion is isoelectronic with
a) \[H^{+}\]
b) \[He^{+}\]
c) He
d) Be
Discussion
Explanation: Hydride ion H- contains two electrons and He contains two electrons.
3. Which of the following isoelectronic species has the smallest atomic radius?
a) \[N^{3-}\]
b) \[O^{2-}\]
c) \[F^{-}\]
d) Ne
Discussion
Explanation: \[F^{-}\]
4. Among the following groupings which represents the collection of isoelectronic species?
a) \[NO^{+},C_2^{2-},O_2^-,CO\]
b) \[N_{2},C_2^{2-},CO,NO\]
c) \[CO,NO^{+},CN^{-},C_2^{2-}\]
d) \[NO,CN^{-},N_{2},O_2^-\]
Discussion
Explanation: The species \[CO,NO^{+},CN^{-},C_2^{2-}\] Contain 14 electrons each.
5. Which of the following is not iso electronic
a) \[Na^{+}\]
b) \[Mg^{2+}\]
c) \[O^{2-}\]
d) \[Cl^{-}\]
Discussion
Explanation: Na+ , Mg++, O2- contain 10 electrons each Cl- has 18 electrons.
6. Chloride ion and potassium ion are isoelectronic. Then
a) their sizes are same
b) chloride ion is bigger than potassium ion
c) potassium ion is relatively bigger
d) depends upon the other cation or anion
Discussion
Explanation: Nuclear charge of K+ (19) is more than nuclear charge of Cl– (17).
7. Ratio of energy of photon of wavelength 3000 Å and 6000Å is
a) 3 : 1
b) 2 : 1
c) 1 : 2
d) 1 : 3
Discussion
Explanation:
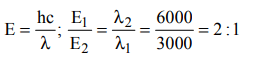
8. The energy of a photon is given as \[\triangle E\diagup atom = 3.03*10^{-19} J atom^{-1}\] . Then the wavelength \[\left(\lambda\right)\] of the photon is.
a) 65.6 nm
b) 656 nm
c) 0.656 nm
d) 6.56 nm
Discussion
Explanation:
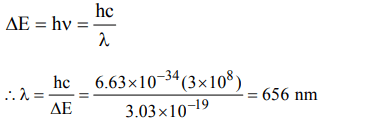
9. If wavelength of photon is \[2.2×10^{-11}m, h=6.6 ×10^{-34}Js\] , then momentum of photon is
a) \[3 × 10^{-23}Kg/s\]
b) \[3.33× 10^{22}Kg/s\]
c) \[1.452× 10^{-44}Kg/s\]
d) \[6.89× 10^{43}Kg/s\]
Discussion
Explanation:
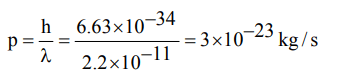
10. Which orbital of carbon can absorb photon but not emit it?
a) 1 s
b) 2 s
c) 3 p
d) 2 p
Discussion
Explanation: It is in the lowest energy level, it can absorb and not emit energy.