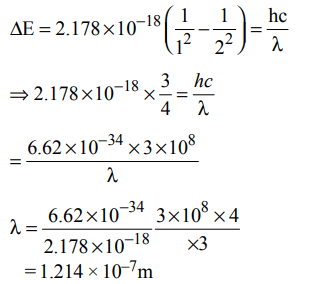1.The ionization enthalpy of hydrogen atom is \[1.312 ×10^{6}J mol^{-1}\] . The energy required to excite the electron in the atom from n = 1 to n = 2 is
a) \[9.84 ×10^{5}J mol^{-1}\]
b) \[6.56 ×10^{5}J mol^{-1}\]
c) \[7.56 ×10^{5}J mol^{-1}\]
d) \[8.51 ×10^{5}J mol^{-1}\]
Explanation:
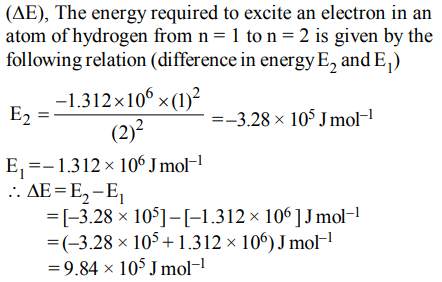
2. Which one of the following constitutes a group of the isoelectronic species?
a) \[C_2^{2-},O_2^-,CO,NO\]
b) \[CN^{-},N_{2},O_2^{2-},C_2^{2-},\]
c) \[NO^{+},C_2^{2-},CN^{-},N_{2}\]
d) \[N_{2},O_2^-,NO^{+},CO\]
Explanation:
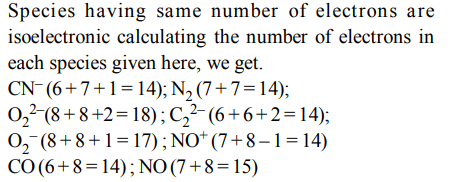
3. Calculate the wavelength (in nanometer) associated with a proton moving at \[1.0 ×10^{3}ms^{-1}\] . (Mass of proton = \[1.67 ×10^{-27}\] Kg and h = \[6.63 × 10^{-34}\] Js)
a) 0.40 nm
b) 2.5 nm
c) 14.0 nm
d) 0.32 nm
Explanation:
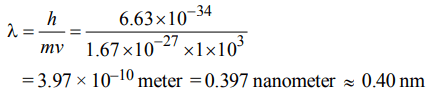
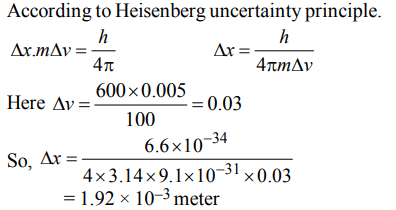
4. In an atom, an electron is moving with a speed of 600 m/s withan accuracy of 0.005%. Certainity with which the position of the electron can be located is \[\left(h = 6.6 × 10^{-34}Kg m^{2}s^{-1},mass of electron,e_{m}=9.1 × 10^{-31}Kg\right)\]
a) \[5.10 × 10^{-3}m\]
b) \[1.92 × 10^{-3}m\]
c) \[3.84 × 10^{-3}m\]
d) \[1.52 × 10^{-4}m\]
Explanation:
5. The energy required to break one mole of Cl – Cl bonds in\[CI_{2}\] is \[242 kJMol^{-1}\] .The longest wavelength of light capable of breaking a single Cl – Cl bond is
\[\left(c = 3 ×10^{8}ms^{-1} andN_{A}=6.02 ×10^{23}mol^{-1}\right)\]
a) 594 nm
b) 640 nm
c) 700 nm
d) 494 nm
Explanation:
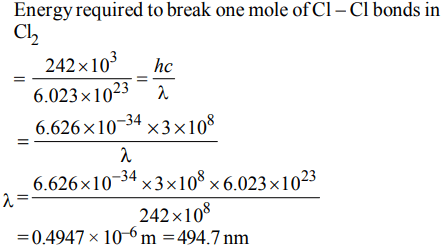
6. Ionisation energy of \[He^{+}\] is \[19.6 × 10^{-18}J atom^{-1}\] . The energy of the first stationary state \[(n = 1) of Li^{2+} \] is
a) \[4.41 × 10^{-16} J atom^{-1}\]
b) \[–4.41 × 10^{-17} J atom^{-1}\]
c) \[–2.2 × 10^{-15} J atom^{-1}\]
d) \[8.82 × 10^{-17} J atom^{-1}\]
Explanation:
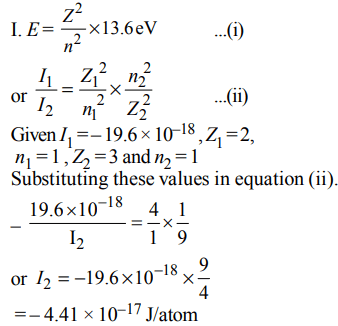
7. The frequency of light emitted for the transition n = 4 to n = 2 of the \[He^{+}\] is equal to the transition in H atom corresponding to which of the following ?
a) n = 2 to n = 1
b) n = 3 to n = 2
c) n = 4 to n = 3
d) n = 3 to n = 1
Explanation:
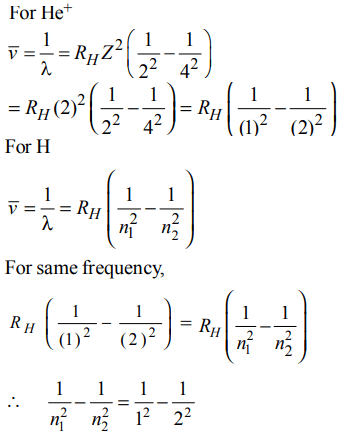
n1 = 1 & n2 = 2
8. The electrons identified by quantum numbers \[n\] and \[\ell\] :
(A) \[n = 4,\ell=1\]
(B) \[n = 4,\ell=0\]
(C) \[n = 3,\ell=2\]
(D) \[n = 3,\ell=1\]
can be placed in order of increasing energy as :
a) (C) < (D) < (B) < (A)
b) (D) < (B) < (C) < (A)
c) (B) < (D) < (A) < (C)
d) (A) < (C) < (B) < (D)
Explanation:
(A) 4 p
(B) 4 s
(C) 3 d
(D) 3 p
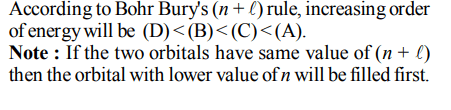
9.The increasing order of the ionic radii of the given isoelectronic species is
a) \[Cl^{-},Ca^{2+},K^{+},S^{2-}\]
b) \[S^{2-},Cl^{-},Ca^{2+},K^{+}\]
c) \[Ca^{2+},K^{+},Cl^{-},S^{2-}\]
d) \[K^{+},S^{2-},Ca^{2+},Cl^{-}\]
Explanation:
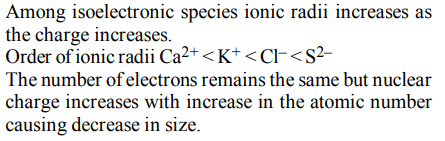
10. Energy of an electron is given by \[E=– 2.178 ×10^{-18}J\left[\frac{Z^{2}}{n^{2}}\right]\] Wavelength of light required to excite an electron in an
hydrogen atom from level n = 1 to n = 2 will be :\[\left(h=6.62 × 10^{-34}Js, c = 3.0 ×10^{8}ms^{-1}\right)\]
a) \[1.214 × 10^{-7}m\]
b) \[2.816 × 10^{-7}m\]
c) \[6.500 × 10^{-7}m\]
d) \[8.500 × 10^{-7}m\]
Explanation: