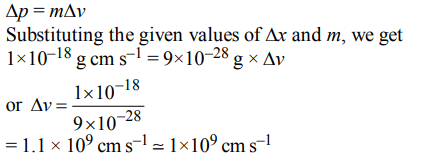1. In the ground state, an element has 13 electrons in its M-shell. The element is
a) zinc
b) chromium
c) nickel
d) iron
Explanation: M-shell means 3 principle energy level the element is Cr
2. Which one of the following pairs of ions has the same electronic configuration?
a) \[Cr^{3+},Fe^{3+}\]
b) \[Fe^{3+},Mn^{2+}\]
c) \[Fe^{3+},Co^{3+}\]
d) \[Sc^{3+},Cr^{3+}\]
Explanation: \[Fe^{3+},Mn^{2+}\]
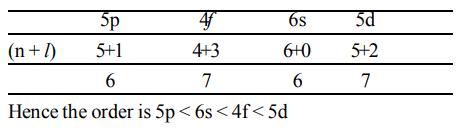
3. The correct order of increasing energy of atomic orbitals is
a) 5 p < 4 f < 6 s < 5 d
b) 5 p < 6 s < 4 f < 5 d
c) 5 p < 5 d < 4 f < 6 s
d) none of these
Explanation:
4.The number of d-electrons retained in \[Fe^{2+}\] (At. no. of Fe = 26) ion is
a) 3
b) 4
c) 5
d) 6
Explanation: Fe2+ 1s2 , 2s2p6 , 3s2p6d6 hence 6 electrons
5. The correct electronic configuration of Cu (29) is
a) \[1s^{2}2s^{2}2p^{6}3s^{2}3p^{6}3d^{10}4s^{1}\]
b) \[1s^{2}2s^{2}2p^{6}3s^{2}3p^{6}3d^{6}\]
c) \[1s^{2}2s^{2}2p^{6}3s^{1}3p^{3}3d^{10}\]
d) \[1s^{2}2s^{2}2p^{6}3s^{2}3p^{6}3d^{4}4s^{2}\]
Explanation: \[1s^{2}2s^{2}2p^{6}3s^{2}3p^{6}3d^{10}4s^{1}\]
6. The paramagnetic character follows the order
a) Mn > Cr > Zn
b) Fe > Zn > Co
c) Cr > Fe > Zn
d) Hg > Mn > Fe
Explanation: Cr (6), Fe (4), Mn (5), Cu (3), Zn (0), Hg (0). The more the number of unpaired electrons the more is the paramagnetic character.
7. If the nitrogen atom had electronic configuration \[1s^{7}\] it would have energy lower than that of the normal ground state configuration \[1s^{2}2s^{2}2p^{3}\] because the electrons would be closer to the nucleus. Yet \[1s^{7}\] is not observed. It violates
a) Heisenberg’s uncertainty principle
b) Hund’s rule
c) Pauli exclusion principle
d) Bohr postulate of stationary orbits
Explanation: Not more than two electrons can be present in same atomic orbital. This is Paulis exclusion principle.
8. Which of the following elements outermost orbit’s last electron has magnetic quantum number m = 0?
a) Na
b) O
c) Cl
d) N
Explanation: \[_{11}Na\] = 1s2 , 2s2p6 , 3s1 for 3s1 , l = 0. Hence m = 0.
9. If uncertainty in position and momentum are equal, then uncertainty in velocity is :
a) \[\frac{1}{2 m}\sqrt{\frac{h}{\pi}}\]
b) \[\sqrt{\frac{h}{2\pi}}\]
c) \[\frac{1}{ m}\sqrt{\frac{h}{\pi}}\]
d) \[\sqrt{\frac{h}{\pi}}\]
Explanation:
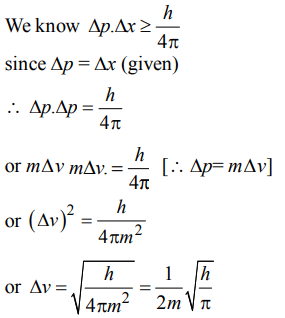
10. The measurement of the electron position is associated with an uncertainty in momentum, which is equal to \[1×10^{-18}g cms^{-1}\] . The uncertainty in electron velocity is,(mass of an electron is \[9×10^{-28}g\] )
a) \[1×10^{9} cms^{-1}\]
b) \[1×10^{6} cms^{-1}\]
c) \[1×10^{5} cms^{-1}\]
d) \[1×10^{11} cms^{-1}\]
Explanation: