1. A body of mass 10 mg is moving with a velocity of \[100 ms^{-1}\] . The wavelength of de-Broglie's wave associated with it would be
a) \[6.63 ×10^{-35}m\]
b) \[6.63 ×10^{-31}m\]
c) \[6.63 ×10^{-37}m\]
d) \[6.63 ×10^{-34}m\]
Explanation:
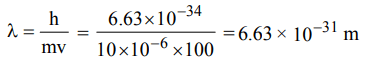
2. If \[N_{0}\] represents the Avogadro number, then which of the following represent correct value of one atomic mass unit.
a) \[N_{0}*10^{-3} kg\]
b) \[N_{0}g\]
c) \[N_0^{-1}g\]
d) \[\frac{1}{16}\] Mass of 0-16 atom
Explanation:
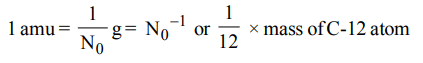
3. If the shortest wavelength of the spectral line of H-atom in the Lyman series is X, then the longest wavelength of the line in Balmer series of \[Li^{2+}\] is
a) 9x
b) \[\frac{x}{9}\]
c) \[\frac{5x}{4}\]
d) \[\frac{4x}{5}\]
Explanation:
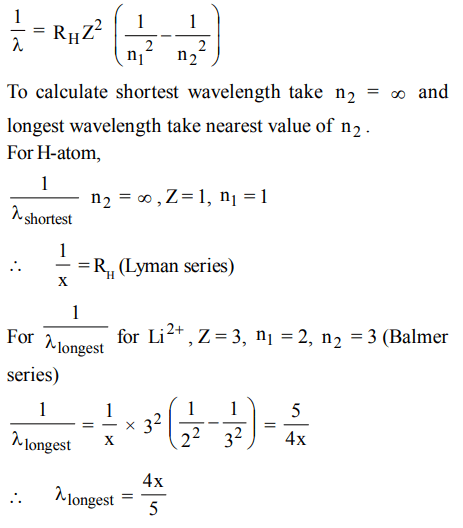
4. If the subsidiary quantum number of a sub-energy level is 4, the maximum and minimum values of the spin multiplicities are
a) 10, 2
b) 4, – 4
c) 10, 1
d) 9, 1
Explanation:
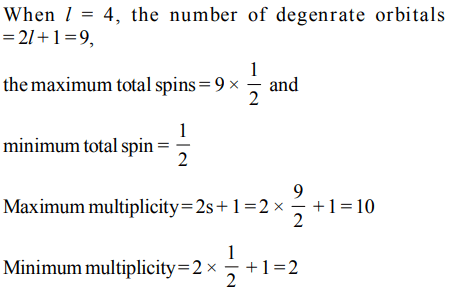
5. \[Li^{3+}\] and a proton are accelerated by the same potential, then de-Broglie wavelengths \[\lambda_{Li}and \lambda_{p}\] have the ratio
assume \[\left(m_{Li}=9m_{p}\right)\]
a) \[1 :3\sqrt{3}\]
b) 1 : 1
c) 1 : 2
d) 1 : 4
Explanation:
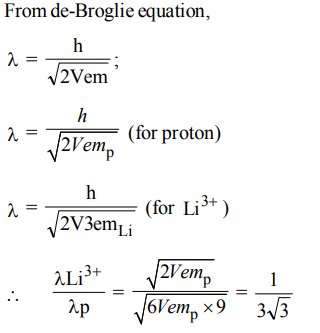
6. One Bohr magnet on is equal to
a) \[\frac{c}{4\pi hm_{e}}\]
b) \[\frac{h}{4\pi em_{e}}\]
c) \[\frac{eh}{4\pi m_{e}}\]
d) \[\frac{hc}{4\pi m_{e}}\]
Explanation:
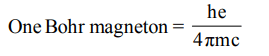
7. The dissociation energy of H2 is \[ 430.53 KJ mol^{-1}\] . If hydrogen is dissociated by illumination with radiation of wavelength 253.7 nm the fraction of the radiant energy which
will be converted into kinetic energy is given by
a) 100%
b) 8.76%
c) 2.22%
d) 1.22%
Explanation:
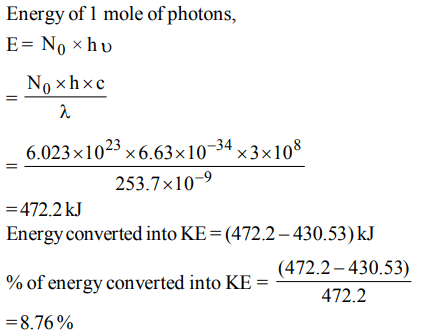
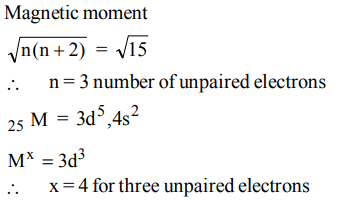
8. The magnetic moment of \[ M^{x+}\](atomic number M = 25) is \[\sqrt{15}BM\]. The number of unpaired elections and the value of x respectively are
a) 5, 2
b) 3, 2
c) 3, 4
d) 4, 3
Explanation:
9. The threshold frequency of a metal is \[1 ×10^{15}s^{-1}\] . The ratio of the maximum kinetic energies of the photoelectrons when the metal is irradiated with radiations of frequencies
\[1.5 ×10^{15}s^{-1}, 2.0 ×10^{15}s^{-1}\] respectively would be
a) 4 : 3
b) 1 : 2
c) 2 : 1
d) 3 : 4
Explanation:
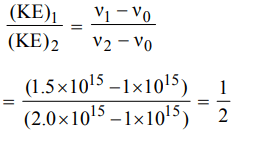
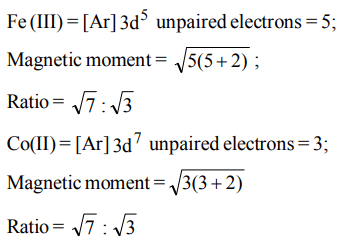
10. The ratio of magnetic moments of Fe(III) and Co(II) is
a) 7 : 3
b) 3 : 7
c) \[\sqrt{7}: \sqrt{3}\]
d) \[\sqrt{3}: \sqrt{7}\]
Explanation: