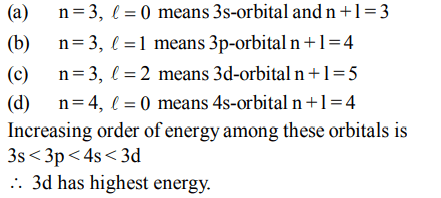1. Consider the ground state of Cr atom (X = 24). The number of electrons with the azimuthal quantum numbers, l = 1 and 2
are, respectively
a) 16 and 4
b) 12 and 5
c) 12 and 4
d) 16 and 5
Explanation:
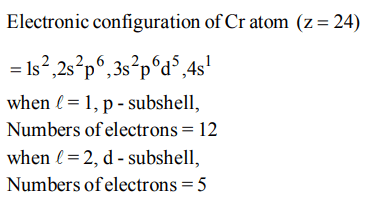
2. The wavelength of the radiation emitted, when in a hydrogen atom electron falls from infinity to stationary state 1, would be\[\left(Rydberg constant = 1.097×10^{7}m^{-1}\right)\]
a) 406 nm
b) 192 nm
c) 91 nm
d) \[9.1×10^{-8}nm\]
Explanation:
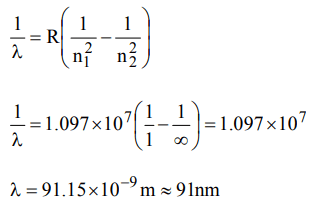
3. Which one of the following sets of ions represents the collection of isoelectronic species?
a) \[K^{+},Cl^{-},Mg^{2+},Sc^{3+}\]
b) \[Na^{+},Ca^{2+}, Sc^{3+},F^{-}\]
c) \[K^{+},Ca^{2+}, Sc^{3+},Cl^{-}\]
d) \[Na^{+},Mg^{2+}, AI^{3+},Cl^{-}\]
Explanation: \[_{19}K^+\], \[_{20}Ca^{2+}\], \[_{21}Sc^{3+}\], \[_{17}Cl^-\] each contains 18 electrons
4. Of the following outer electronic configurations of atoms, the highest oxidation state is achieved by which one of them ?
a) \[\left(n-1\right)d^{3}ns^{2}\]
b) \[\left(n-1\right)d^{5}ns^{1}\]
c) \[\left(n-1\right)d^{8}ns^{2}\]
d) \[\left(n-1\right)d^{5}ns^{2}\]
Explanation: (n–1)d5ns2 attains the maximum O.S. of + 7
5. In a multi-electron atom, which of the following orbitals described by the three quantum members will have the same energy in the absence of magnetic and electric fields ?
(A) n = 1, l = 0, m = 0
(B) n = 2, l = 0, m = 0
(C) n = 2, l = 1, m = 1
(D) n = 3, l = 2, m = 1
(E) n = 3, l = 2, m = 0
a) (D) and (E)
b) (C) and (D)
c) (B) and (C)
d) (A) and (B)
Explanation: The energy of an orbital is given by (n + l) in (d) and (c). (n + l) value is (3 + 2) = 5 hence they will have same energy.
6. Of the following sets which one does NOT contain isoelectronic species?
a) \[BO_3^{3-},CO_3^{2-},NO_3^-\]
b) \[SO_3^{2-},CO_3^{2-},NO_3^-\]
c) \[CN^{-},N_{2},C_2^{2-}\]
d) \[PO_3^{4-},SO_4^{2-},CIO_4^-\]
Explanation:
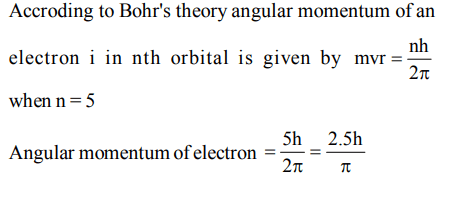
7. According to Bohr's theory, the angular momentum of an electron in 5th orbit is
a) \[10 h/\pi\]
b) \[2.5 h/\pi\]
c) \[25 h/\pi\]
d) \[1.0 h/\pi\]
Explanation:
8. Uncertainty in the position of an electron \[\left(mass=9.1 ×10^{-31}kg\right)\] moving with a velocity \[300ms^{-1}\] , accurate upto
0.001% will be \[\left(h = 6.63 × 10^{-34}Js\right)\]
a) \[1.92 × 10^{-2}m\]
b) \[3.84 × 10^{-2}m\]
c) \[19.2 × 10^{-2}m\]
d) \[5.76× 10^{-2}m\]
Explanation:
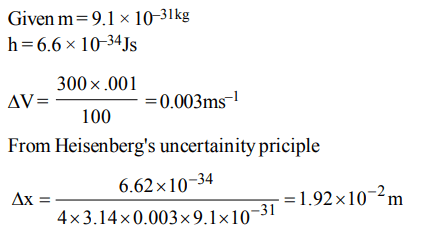
9. Which one of the following sets of ions represents a collection of isoelectronic species?
a) \[N^{3-},O^{2-},F^{-},S^{2-}\]
b) \[Li^{+},Na^{+},Mg^{2+},Ca^{2+}\]
c) \[K^{+},Cl^{-},Ca^{2+},SC^{3+}\]
d) \[Ba^{2+},Sr^{2+},K^{+},Ca^{2+}\]
Explanation:
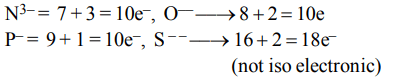
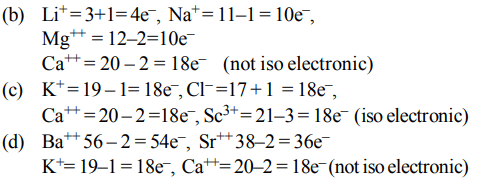
10. Which of the following sets of quantum numbers represents the highest energy of an atom?
a) n = 3, l = 0, m = 0, s = +1/2
b) n = 3, l = 1, m = 1, s = +1/2
c) n = 3, l = 2, m = 1, s = +1/2
d) n = 4, l = 0, m = 0, s = +1/2.
Explanation: