1. The number of nodal planes ‘d’ orbital has
a) 1
b) 2
c) 3
d) 0
Explanation: Number of nodal planes in d orbitals is 2
2. What do you mean by degenerate orbitals?
a) Orbitals having equal energy
b) Oribitals having equal wave function
c) Oribitals having equal energy but different wave function
d) Orbitals having equal energy and equal wave function
Explanation: Degenerate orbitals have equal energy
3. The number of nodal planes in a \[p_{X}\] orbital is
a) one
b) two
c) three
d) zero
Explanation: One nodal plane in the YZ plane
4. A 5f orbital has
a) one node
b) two nodes
c) three nodes
d) four nodes
Explanation: Angular nodes = l, spherical nodes (n – l – 1); Total (n – 1). Hence spherical nodes for 5f orbits. = ( 5 –3 –1) = 1
5. “ No two electrons in an atom can have same set of all the four quantum numbers” is known as
a) Hund’s rule
b) Aufbau principle
c) Uncertainty principle
d) Pauli’s exclusion principle
Explanation: Pauli’s exclusion principle
6. Which of the following has maximum number of unpaired electrons
a) \[Mg^{2+}\]
b) \[Ti^{3+}\]
c) \[V^{3+}\]
d) \[Fe^{2+}\]
Explanation:
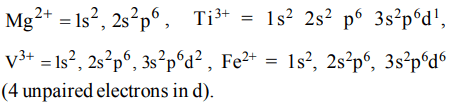
7. An element M has an atomic mass 19 and atomic number 9, its ion is represented by
a) \[M^{+}\]
b) \[M^{2+}\]
c) \[M^{-}\]
d) \[M^{2-}\]
Explanation: Atomic number 9 is for F and ion is F–
8. The configuration \[1s^{2}2s^{2}2p^{5}3 s^{1}\] shows
a) ground state of fluorine
b) excited state of fluorine
c) excited state of neon atom
d) excited state of \[O_2^-\] ion
Explanation: 1s2 , 2s22p5 , 3s1 . Total electrons (10) excited state of Ne
9. Heisenberg's Uncertainity principle is applicable to
a) atoms only
b) electron only
c) nucleus only
d) any moving object
Explanation: Heisenberg's uncertainty Principle is applicable to any moving object
10. Electronic configuration of four elements are given below. Which of the corresponding element would be most paramagetic?
a) \[1s^{2}2s^{2}2p^{6}\]
b) \[1s^{2}2s^{2}2p^{1}\]
c) \[1s^{2}2s^{2}2p^{5}\]
d) \[1s^{2}2s^{2}2p^{4}\]
Explanation: Configuration contains 2 unpaired electrons hence most paramagnetic