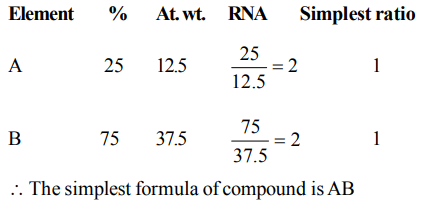1. In which of the following number all zeros are significant?
a) 0.0005
b) 0.0500
c) 50.000
d) 0.0050
Explanation: If zero is used to locate the decimal point it is considered a significant figure. In 50.000 all zero are significant
2. If law of conservation of mass was to hold true, then 20.8 gm of \[BaCI_{2}\] on reaction with 9.8 gm of \[H_{2}SO_{4}\] will produce 7.3 gm of HCl and \[BaCI_{4}\] equal to :
a) 11.65 gm
b) 23.3 gm
c) 25.5 gm
d) 30.6 gm
Explanation:
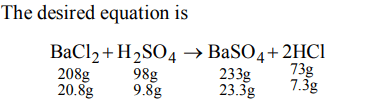
3. One of the following combination which illustrates the law of reciprocal proportions ?
a) \[N_{2}O_{3},N_{2}O_{4},N_{2}O_{5}\]
b) NaCl, NaBr, NaI
c) \[CS_{2},CO_{2},SO_{2}\]
d) \[PH_{3},P_{2}O_{3},P_{2}O_{5}\]
Explanation: In law of reciprocal proportions, the two elements combining with the third element, must combine with each other in the same ratio or multiple of that Ratio of S and O when combine with C is 2 : 1. Ratio of S and O is SO2 is 1 : 1
4. If isotopic distribution of C-12 and C-14 is 98% and 2% respectively then the no. of C-14 atoms in 12gm of carbon is
a) \[1.032*10^{22}\]
b) \[3.0*10^{22}\]
c) \[5.88*10^{23}\]
d) \[6.02*10^{23}\]
Explanation:
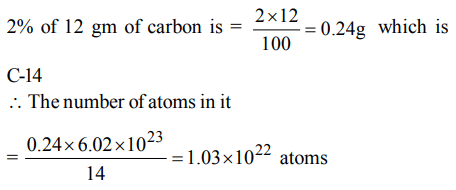
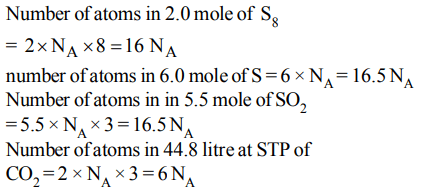
5. Which of the following contains maximum number of atom
a) \[2.0\] mole of \[S_{8}\]
b) 6.0 mole of S
c) \[5.5\] mole of \[SO_{2}\]
d) \[44.8\] litre of \[CO_{2}\] of S.T.P.
Explanation:
6. A sample of \[AIF_{3}\] contains \[3.0*10^{24}F^{-}\] ions. The number of formula unit of this sample are
a) \[9*10^{24}\]
b) \[3*10^{24}\]
c) \[0.75*10^{24}\]
d) \[1.0*10^{24}\]
Explanation:

7. What mass of calcium chloride in grams would be enough to produce 14.35 gm of AgCl ?
a) 5.55 gm
b) 8.295 gm
c) 16.5 gm
d) 11.19 gm
Explanation:
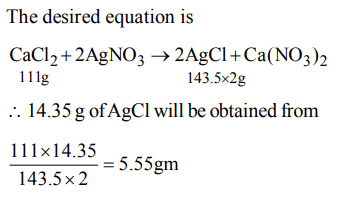
8. If potassium chlorate is 80% pure, then 48 gm of oxygen would be produced from (atomic mass of K =39)
a) \[153.12\] gm of \[KCIO_{3}\]
b) \[122.5\] gm of \[KCIO_{3}\]
c) \[245\] gm of \[KCIO_{3}\]
d) \[98\] gm of \[KCIO_{3}\]
Explanation:
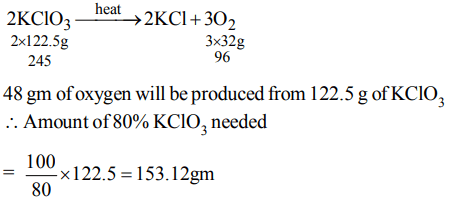
9. If 224 ml of a triatomic gas has a mass of 1 gm at 273K and 1 atmospheric pressure then the mass of one atom is
a) \[8.30 ×10^{-23}\]
b) \[2.08 ×10^{-23}\]
c) \[5.53 ×10^{-23}\]
d) \[6.24 ×10^{-23}\]
Explanation:
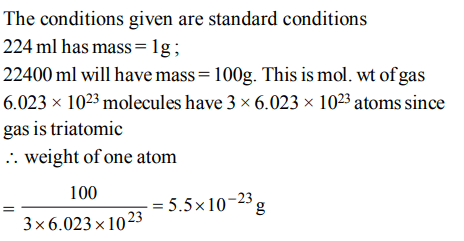
10. A compound made up of two elements A and B is found to contain 25% A (atomic mass = 12.5) and 75% B (atomic mass
= 37.5). The simplest formula of the compound is
a) AB
b) \[AB_{2}\]
c) \[AB_{3}\]
d) \[A_{3}B\]
Explanation: