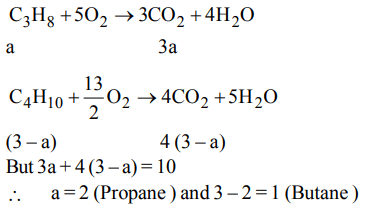1. If \[3.01 ×10^{20}\] molecules are removed from 98 mg of \[H_{2}SO_{4}\] , then the number of moles of \[H_{2}SO_{4}\] left are
a) \[0.1 ×10^{-3}\]
b) \[0.5 ×10^{-3}\]
c) \[1.66 ×10^{-3}\]
d) \[9.95 ×10^{-2}\]
Explanation:

2. 25.4 g of \[I_{2}\] and 14.2 g of \[Cl_{2}\] are made to react completely to yield a mixture of \[ICl\] and \[ICl_{3}\] . Calculate moles of \[ICl\] and \[ICl_{3}\] formed
a) 0.1, 0.1
b) 0.2, 0.2
c) 0.1, 0.2
d) 0.2, 0.1
Explanation:
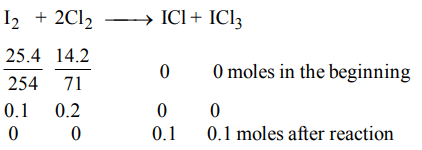
3. 2 g of a mixture of \[CO and CO_{2}\] on reaction with excess I2O5 produced \[2.54 g of I_{2}\] . What be the mass % of \[CO_{2}\] in the original mixture ?
a) 35
b) 70
c) 30
d) 90
Explanation:

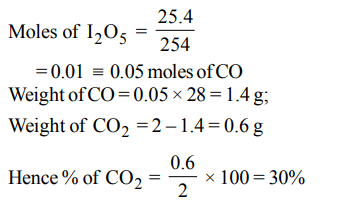
4. The hydrated salt \[Na_{2}CO_{3}.xH_{2}O\] undergoes 63% loss in mass on heating and becomes anhydrous. The value of x is
a) 10
b) 7
c) 5
d) 3
Explanation:

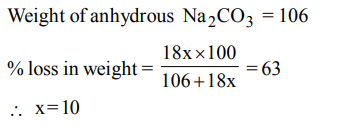
5. Gastric juice contains 3.0 g of HCl per litre. If a person produces 2.5 litre of gastric juice per day. How many antacid tablets each containing 400 mg of \[Al\left(OH\right)_{3}\] are needed to neutralize all the HCl produced in one day ?
a) 18
b) 14
c) 20
d) 17
Explanation:
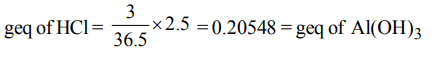
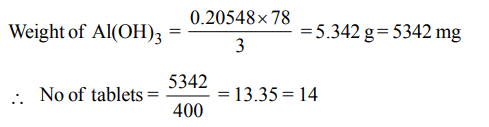
6. Sulfuryl chloride \[\left(SO_{2}CI_{2}\right)\] reacts with water to give a mixture of \[H_{2}SO_{4}\] and HCl. How many moles of baryta would be required to neutralize the solution formed by adding
4 mol of \[SO_{2}CI_{2}\] to excess of water ?
a) 1
b) 2
c) 3
d) 4
Explanation:
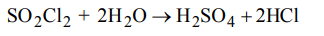
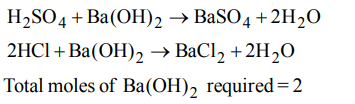
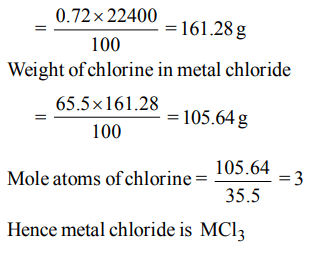
7. A chloride of a metal (M) contain 65.5% of chlorine. 100 ml of vapour of the chloride of metal at STP weights 0.72 g. The molecular formula of the metal chloride is
a) \[MCl_{4}\]
b) \[MCl_{3}\]
c) \[MCl_{2}\]
d) MCl
Explanation: Molecular weight of metal chloride
8. 7.36 g of a mixture of KCl and KI was dissolved in \[H_{2}O\] to prepare 1 litre solution. 25 ml of this required 8.45 ml of 0.2 N \[AgNO_{3}\] , what are % of KI in mixture ?
a) 57.28
b) 47.28
c) 5.72
d) 49.12
Explanation:
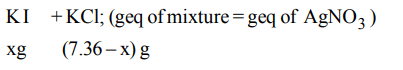
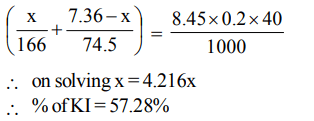
9. When burnt in air, 14.0 g mixture of carbon and sulphurgives a mixture of \[CO_{2} and SO_{2}\] in the volume ratio of 2 : 1, volume being measured at the same conditions of temperature and pressure moles of carbon in the mixture is
a) 0.75
b) 0.5
c) 0.40
d) 0.25
Explanation:
10. A gas mixture of 3 litres of propane \[\left(C_{3}H_{8}\right)\] and butane \[\left(C_{4}H_{10}\right)\] on complete combustion at 25° C produced 10
litre \[CO_{2}\] . Find out the composition of gas mixture (Propane : Butane)
a) 2 : 1
b) 1 : 2
c) 1.5 : 1.5
d) 0.5 : 2.5
Explanation: