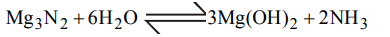1. An organic compound contains carbon, hydrogen and oxygen. Its elemental analysis gave C, 38.71% and H, 9.67%. The empirical formula of the compound would be :
a) \[CH_{3}O\]
b) \[CH_{2}O\]
c) CHO
d) \[CH_{4}O\]
Explanation:
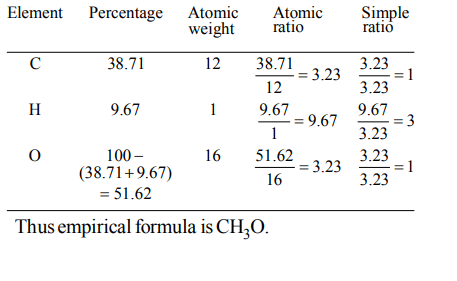
2. How many moles of lead (II) chloride will be formed from a reaction between 6.5 g of PbO and 3.2 g of HCl ?
a) 0.044
b) 0.333
c) 0.011
d) 0.029
Explanation:
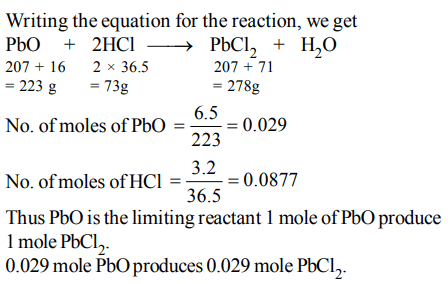
3. 10 g of hydrogen and 64 g of oxygen were filled in a steel vessel and exploded. Amount of water produced in this reaction will be:
a) 3 mol
b) 4 mol
c) 1 mol
d) 2 mol
Explanation:
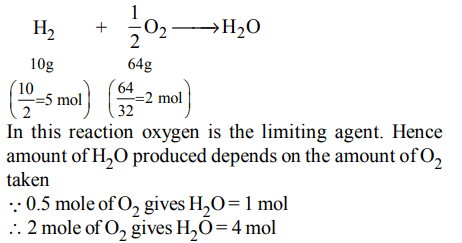
4. The number of atoms in 0.1 mol of a triatomic gas is :\[\left(N_{A}=6.02 ×10^{23}mol^{-1}\right)\]
a) \[6.026 ×10^{22}\]
b) \[1.806 ×10^{23}\]
c) \[3.600 ×10^{23}\]
d) \[1.800 ×10^{22}\]
Explanation: The number of atoms in 0.1 mole of a triatomic gas
= 0.1 × 3 × 6.023 × 1023
= 1.806 × 1023
5. Which has the maximum number of molecules among the following ?
a) 44 g \[CO_{2}\]
b) 48 g O3
c) 8 g \[H_{2}\]
d) 64 g \[SO_{2}\]
Explanation:
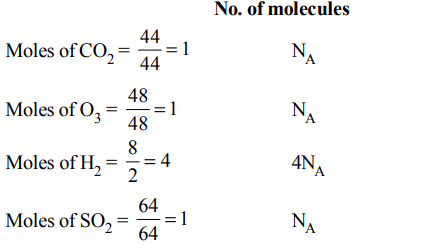
6. How many grams of concentrated nitric acid solution should be used to prepare 250 mL of 2.0M \[HNO_{3}\] ? The concentrated acid is 70% \[HNO_{3}\]
a) \[90.0 g conc.HNO_{3}\]
b) \[70.0 g conc.HNO_{3}\]
c) \[54.0 g conc.HNO_{3}\]
d) \[45.0 g conc.HNO_{3}\]
Explanation:
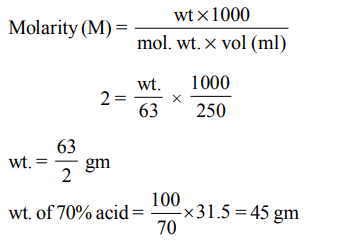
7. \[6.02 ×10^{20}\] molecules of urea are present in 100 mL of its solution. The concentration of solution is :
a) 0.01 M
b) 0.001 M
c) 0.1 M
d) 0.02 M
Explanation:
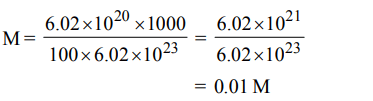
8. With increase of temperature, which of these changes?
a) molality
b) weight fraction of solute
c) fraction of solute present in water
d) mole fraction
Explanation: Volume increases with rise in temperature. Thus, some water molecule may be evaporated at high temp.
9. Number of atoms in 558.5 gram Fe \[\left(at. wt. of Fe = 55.85g mol^{-1}\right)\] is
a) twice that in 60 g carbon
b) \[6.023*10^{22}\]
c) half that in 8 g He
d) \[558.5 *6.023*10^{23}\]
Explanation: Fe (no. of moles) = \[\frac{558.5}{55.85}\] = 10 moles
C (no. of moles) = 60/12 = 5 moles.
10. One mole of magnesium nitride on the reaction with an excess of water gives :
a) two moles of ammonia
b) one mole of nitric acid
c) one mole of ammonia
d) two moles of nitric acid
Explanation: