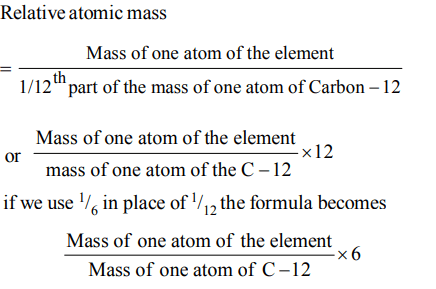
1. If we consider that 1/6, in place of 1/12, mass of carbon atom is taken to be the relative atomic mass unit, the mass of one mole of the substance will
a) be a function of the molecular mass of the substance
b) remain unchanged
c) increase two fold
d) decrease twice
Explanation:
2. How many moles of magnesium phosphate, \[Mg_{3}\left(PO_{4}\right)_{2}\] will contain 0.25 mole of oxygen atoms?
a) \[1.25 ×10^{-2}\]
b) \[2.5 ×10^{-2}\]
c) 0.02
d) \[3.125 ×10^{-2}\]
Explanation:
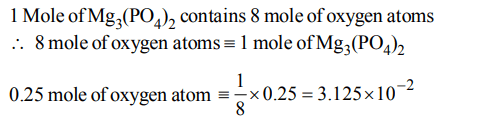
3. The density \[\left(in g mL^{-1}\right)\] of a 3.60 M sulphuric acid solution that is 29% \[H_{2}SO_{4}\left(molar mass = 98 g mol^{-1}\right)\] by mass will be
a) 1.45
b) 1.64
c) 1.88
d) 1.22
Explanation:

4. In the reaction, \[2AI\left(s\right)+6HCl\left(aq\right)\rightarrow2Al^{3+}\left(aq\right)+6CI^{-}\left(aq\right)+3H_{2}\left(g\right)\]
a) 11.2 L \[H_{2}\left(g\right)\] at STP is produced for every mole \[HCI\left(aq\right)\] consumed
b) 6 L \[ HCl\left(aq\right)\] is consumed for every 3 L \[H_{2}\left(g\right)\] produced
c) 33.6 L \[H_{2}\left(g\right)\] is produced regardless of temperature and pressure for every mole Al that reacts
d) \[67.2 H_{2}\left(g\right)\] at STP is produced for every mole Al that reacts.
Explanation:
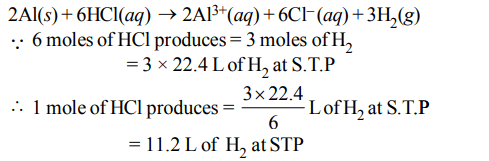
5. The molality of a urea solution in which 0.0100 g of urea, \[\left[\left(NH_{2}\right)_{2}CO\right]\] is added to \[0.3000 dm^{3}\] of water at STP is :
a) \[5.55*10^{-4}\] m
b) 33.3 m
c) \[3.33*10^{-2}\] m
d) 0.555 m
Explanation:
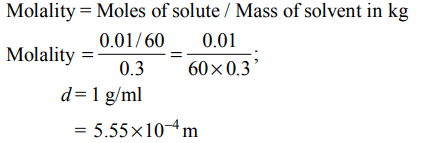
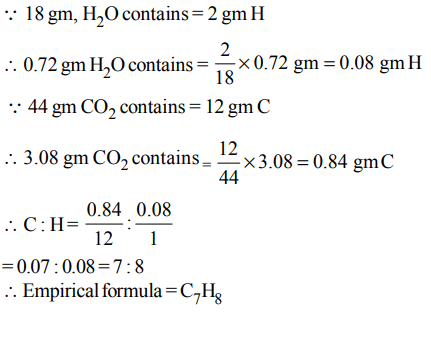
6. A gaseous hydrocarbon gives upon combustion 0.72 g of water and 3.08 g. of \[CO_{2}\]. The empirical formula of the hydrocarbon is :
a) \[C_{2}H_{4}\]
b) \[C_{3}H_{4}\]
c) \[C_{6}H_{5}\]
d) \[C_{7}H_{8}\]
Explanation:
7. Experimentally it was found that a metal oxide has formula \[M_{0.98}O\] . Metal M, present as \[M^{2+} and M^{3+}\] in its oxide. Fraction of the metal which exists as \[M^{3+} \] would be :
a) 7.01%
b) 4.08%
c) 6.05%
d) 5.08%
Explanation:
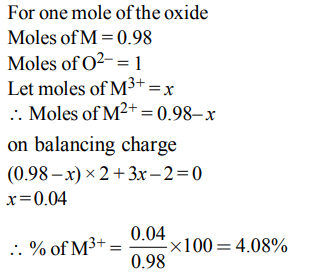
8. Consider a titration of potassium dichromate solution with acidified Mohr's salt solution using diphenylamine as indicator. The number of moles of Mohr's salt required per mole of dichromate is
a) 3
b) 4
c) 5
d) 6
Explanation:

9. The correctly reported answer of addition of 29.4406, 3.2 and 2.25 will have significant figures
a) 3
b) 4
c) 2
d) 5
Explanation: Sum of the figures 29.4406, 3.2 and 2.25 is 34.8906. The sum should be reported to the first place of decimal as 3.2 has only one decimal place. After rounding off the sum is 34.9. Hence number of significant figures is three.
10. On dividing 0.25 by 22.1176 the actual answer is 0.011303. The correctly reported answer will be
a) 0.011
b) 0.01
c) 0.0113
d) 0.013
Explanation: 0.25/22.1176 = 0.011303. The least precise term has two significnat figures, hence the result is 0.011