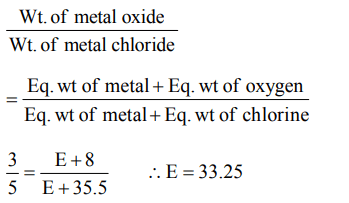1.7.5 grams of a gas occupy 5.6 litres of volume at STP. The gas is
a) \[N_{2}O\]
b) NO
c) CO
d) \[CO_{2}\]
Explanation:
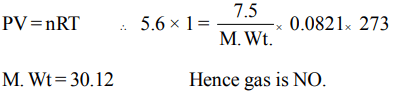
2. 1 amu is equal to
a) \[\frac{1}{14} of O-16\]
b) \[\frac{1}{12} of C-12\]
c) \[1g of H_{2}\]
d) \[1.66 × 10^{-23}kg\]
Explanation: 1 amu = \[\frac{1}{12}\] of the mass of C-12
3. Which of the following contains maximum number of atoms?
a) \[6.023 × 10^{21}molecules ofCO_{2}\]
b) 0.44 g of \[CO_{2}\]
c) 22.4 L of \[CO_{2}\] at STP
d) None of these
Explanation: 22.4 L of CO2 at STP = 1 mole = 6.023 × 1023 molecules. Hence number of atoms 3 × 6.023 × 1023
4. Number of g of oxygen in 32.2 g \[Na_{2}SO_{4}.10H_{2}O\] is
a) 20.8
b) 2.24
c) 22.4
d) 2.08
Explanation: M. Wt of Na2SO4 .10 H2O is 322 g which contains 224 g oxygen.
32.2 g will contain 22.4 g oxygen
5. The specific heat of a metal is 0.16, its approximate atomic weight would be
a) 32
b) 16
c) 64
d) 40
Explanation:
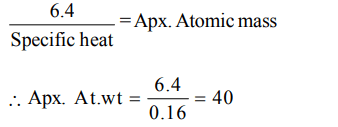
6. The weight of a molecule of the compound \[C_{60}H_{122}\] is
a) \[1.09 × 10^{-21}g\]
b) \[1.4 × 10^{-21}g\]
c) \[5.025 × 10^{23}g\]
d) \[16.023 × 10^{23}g\]
Explanation:
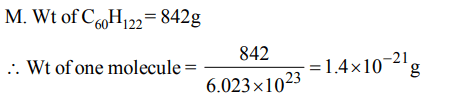
7. The number of water molecules present in a drop of water (volume 0.0018 ml) density = \[18 ml^{-1}\] at room temperature is
a)\[1.084 × 10^{18}\]
b) \[6.023 × 10^{19}\]
c) \[4.84 × 10^{17}\]
d) \[6.023 × 10^{23}\]
Explanation:
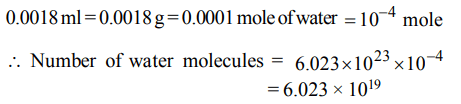
8. The percentage of Se in peroxidase anhydrous enzyme is 0.5% by weight (atomic weight = 78.4). Then minimum molecular weight of peroxidase anhydrous enzyme is
a) \[1.568 × 10^{3}\]
b) \[1.568 × 10^{4}\]
c) 15.68
d) \[3.136 × 10^{4}\]
Explanation:
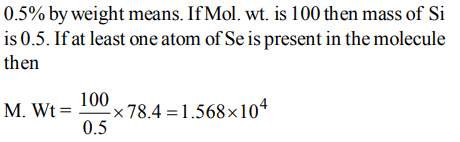
9. Equivalent weight of crystalline oxalic acid is
a) 90
b) 53
c) 63
d) 45
Explanation:
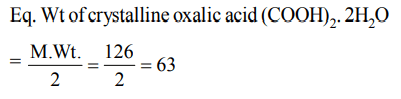
10. 3 g of an oxide of a metal is converted to chloride completely and it yielded 5 g of chloride. The equivalent weight of the metal is
a) 3.325
b) 33.25
c) 12
d) 20
Explanation: