1. Among the following pairs of compounds, the one that illustrates the law of multiple proportions is
a) \[NH_{3}\] and \[NCI_{3} \]
b) \[H_{2}S\] and \[SO_{2} \]
c) \[CS_{2}\] and \[FeSO_{4} \]
d) \[CuO\] and \[Cu_{2}O \]
Explanation: In CuO and Cu2O the O : Cu is 1 : 1 and 1 : 2. This is law of multiple proportion
2. Irrespective of the source, pure sample, of water always yields 88.89% mass of oxygen and 11.11% mass of hydrogen. This is explained by the law of
a) conservation of mass
b) multiple proportions
c) constant composition
d) constant volume
Explanation: The H : O ratio in water is fixed, irrespective of its source. Hence it is law of constant composition.
3. If \[N_{A}\] is Avogadro's number then number of valence electrons in 4.2 g of nitride ions \[\left(N^{3-}\right)\] is
a) \[4.2N_{A}\]
b) \[2.4N_{A}\]
c) \[1.6N_{A}\]
d) \[3.2N_{A}\]
Explanation:

4. Two containers P and Q of equal volume (1 litre each) contain 6 g of \[O_{2}\] and \[SO_{2}\] respectively at 300 K and 1 atmosphere. then
a) Number of molecules in P is less than that in Q
b) Number of molecules in P and Q is same
c) Number of molecules in Q is less than that in P
d) Either (a) or (b)
Explanation:
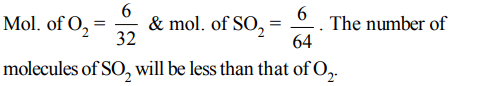
5. The number of moles of oxygen in one litre of air containing 21% oxygen by volume, under standard conditions are
a) 0.0093 mole
b) 0.21 mole
c) 2.10 mole
d) 0.186 mole
Explanation:
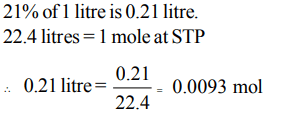
6. The vapour density of a gas is 11.2. The volume occupied by 11.2 g of the gas at NTP will be
a) 22.4 L
b) 11.2 L
c) 1 L
d) 44.8 L
Explanation: V.D. = 11.2
M. Wt = 22.4 g
It corresponds to 22.4 litres at STP
11.2 g = 11.2 L
7. The amount of zinc required to produce 224 ml of \[H_{2} \] at STP on treatment with dil. \[H_{2} SO_{4}\] will be
a) 6.5 g
b) 0.65 g
c) 65 g
d) 0.065 g
Explanation:
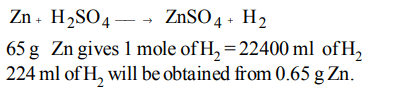
8. The volume occupied by 4.4 g of \[CO_{2} \] at STP is
a) 22.4 L
b) 0.224 L
c) 2.24 L
d) 0.1 L
Explanation: 1 Mol CO2 = 44 g = 22.4 litre at N.T.P.
4.4 g CO2 = 2.24 L at NTP
9. Assuming fully decomposed, the volume of CO2 released at STP on heating 9.85 g of \[BaCO_{3} \] (Atomic mass, Ba = 137) will be
a) 1.12 L
b) 2.24 L
c) 4.06 L
d) 0.84 L
Explanation:
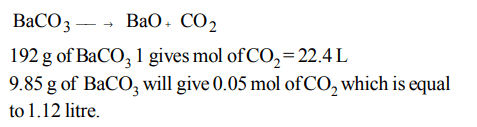
10.\[10 dm^{3} of N_{2}\] gas and \[10 dm^{3}\] of gas X at the same temperature
contain the same number of molecules, the gas X is
a) \[CO_{2}\]
b) CO
c) \[H_{2}\]
d) NO
Explanation: The number of molecules of N2 and X are same. Hence they must have the same molecular weights.
X is CO.