1. The number of molecules in 16 g of methane is
a) \[3.0 × 10^{23}\]
b) \[\frac{16}{6.02} × 10^{23}\]
c) \[6.02 × 10^{23}\]
d) \[\frac{16}{3.0} × 10^{23}\]
Explanation: 16 g CH4 is 1 mol. Hence number of molecules = Avogadro number = 6.023 × 1023
2. 50 ml 10 N \[H_{2}SO_{4}\] , 25 ml 12 N HCl and 40 ml 5 N \[HNO_{3}\] were mixed together and the volume of the mixture was made 1000 ml by adding water. The normality of the resultant solution will be
a) 2 N
b) 1 N
c) 3 N
d) 4 N
Explanation:
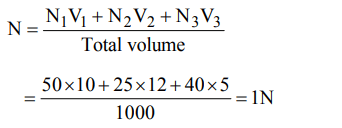
3. A molal solution is one that contains 1 mole of a solute in
a) one litre of the solvent
b) 1000 g of the solvent
c) one litre of the solution
d) 22.4 litres of the solution
Explanation: Molal solution contains 1 mole of solute in 1000 g solvent.
4. A 100 ml solution of 0.1 N HCl was titrated with 0.2 N NaOH solution. The titration was discontinued after adding 30 ml of NaOH solution. The remaining titration was completed
by adding 0.25 N KOH solution. The volume of KOH required for completing the titration is
a) 16 ml
b) 32 ml
c) 35 ml
d) 70 ml
Explanation:
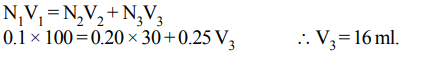
5. An aqueous solution of 6.3 g of oxalic acid dihydrate is made up to 250 ml. The volume of 0.1 N NaOH required to completely neutralise 10 ml of this solution is
a) 20 ml
b) 40 ml
c) 10 ml
d) 4 ml
Explanation:
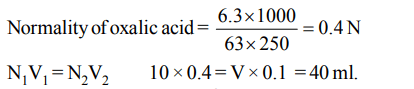
6. The percentage of nitrogen in urea is about
a) 85
b) 46
c) 18
d) 28
Explanation:
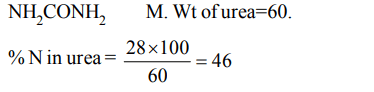
7. How much of NaOH is required to neutralise \[1500cm^{3}\] of 0.1 N HCl? (Na = 23)
a) 60 g
b) 4 g
c) 6 g
d) 40 g
Explanation:
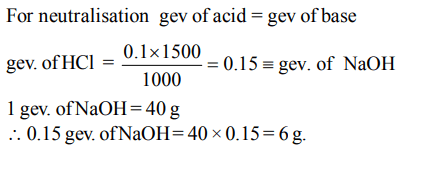
8. The volume of water to be added to \[100 cm^{3}\] of 0.5 N \[H_{2}SO_{4}\] to get deci normal concentration is
a) \[400cm^{3}\]
b) \[450cm^{3}\]
c) \[500cm^{3}\]
d) \[100cm^{3}\]
Explanation:
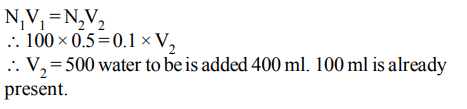
9. 250 ml of a sodium carbonate solution contains 2.65 grams of \[Na_{2}CO_{3}\]. If 10 ml of this solution is diluted to one litre,
what is the concentration of the resultant solution? (mol. wt. of \[Na_{2}CO_{3}=106\] )
a) 0.1 M
b) 0.01 M
c) 0.001 M
d) \[10^{-4}M\]
Explanation:
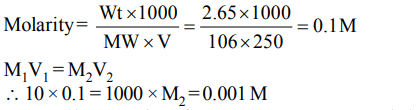
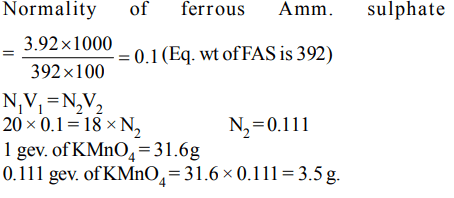
10. 3.92 g of ferrous ammonium sulphate crystals are dissolved in 100 ml of water. 20 ml of this solution requires 18 ml of potassium
permaganate during titration for complete oxidation. The weight of \[KMnO_{4}\] present in one litre of the solution of
a) 3.476 g
b) 12.38 g
c) 1.238 g
d) 34.76 g
Explanation: