1. A balloon is filled with air at a steady rate.” Which process can be correct for this condition?
a) Semi – batch
b) Batch
c) Fed – batch
d) Continuous
Explanation: Semibatch Process is neither batch nor continuous process. A process is operating at a steady state if the values of all the variables in the process do not change with time, except for small fluctuations about a constant mean values. A transient or unsteady-state exists when the process variables change with time. Furthermore, batch and semibatch processes are transient operations, and continuous process can be steady-state or transient.
2. “Chemical reactions or phase changes occur” is this statement applicable for unsteady state energy balance?
a) True
b) False
Explanation: No chemical reactions or phase changes occur as the system is well mixed with uniform temperature and composition. Properties of the outlet stream are therefore the same as within the system and mixtures and solutions are ideal.
3. The exothermic elementary liquid-phase reaction is as below: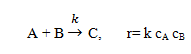
is carried out in a batch reactor with a cooling coil to keep the reactor isothermal at 27°C. The reactor is initially charged with equal concentrations of A and B and no C, cAo = cBo = 2.0 mol/L, cCo = 0.
Additional data:
Rate constant, k = 0.01725 L/mol. min, at 27°C
Heat of reaction, ΔHR = -10 kcal/mol A, at 27°C
Partial molar heat capacities, \(\bar{C}_{PA} = \bar{C}_{PB} \) = 20 cal/mol. K, \(\bar{C}_{PC}\) = 40 cal/mol K
Reactor volume, VR = 1200 L
How long does it take to reach 95% conversion?
a) 550 min
b) 552 min
c) 553 min
d) 551 min
Explanation: Assuming constant density, the material balance for component A is
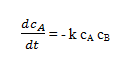
The stoichiometry of the reaction, and the material balance for B gives
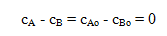
or cA = cB. Substitution into the material balance for species A gives
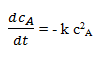
Separation of variables and integration gives
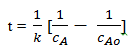
Substituting cA=0.05cAo and the values for k and cAo gives
t = 551 min.
4. Refer to Q3, and estimate what is the total amount of heat (kcal) that must be removed by the cooling coil when this conversion is reached?
Using the following equation:
Q ̇= ΔHR r VR
a) -2.3 × 104 kcal
b) -2.2 × 104 kcal
c) -2.4 × 104 kcal
d) -2.6 × 104 kcal
Explanation: We assume the incompressible-fluid energy balance is accurate for this liquidphase reactor. If the heat removal is manipulated to maintain constant reactor temperature, the time derivative in Equation vanishes leaving
Q ̇ = ΔHR r VR
Substituting dcA/ dt = -r and multiplying through by dt gives
dQ = – ΔHR VR dcA
Integrating both sides gives
Q = – ΔHR VR (cA– cAO) = -2.3 × 104 kcal.
5. Refer to Q3 and Q4 and estimate what is the maximum rate at which heat must be removed by the cooling coil (kcal/min) and at what time does this maximum occur?
Using the following equation:
Q ̇ = ΔHR kc2Ao VR
a) −828 kcal/min
b) –880 kcal/min
c) -825 kcal/min
d) – 850 kcal/min
Explanation: The right-hand side is a maximum in absolute value (note it is a negative quantity) when cA is a maximum, which occurs for cA = cAo, giving
Qmax = ΔHR kc2Ao VR = (−10 kcal/mol A) (0.01725 L/mol•min) (2.0 mol/L )2 (1200 L)
= −828 kcal/min.
6. Refer to Q3, Q4 and Q5 estimate what is the adiabatic temperature rise for this reactor and what is its significance?
Using the equation: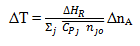
a) 230 K
b) 200 K
c) 250 K
d) 260 K
Explanation: The maximum temperature rise corresponds to complete conversion of the reactants and can be computed from the given data
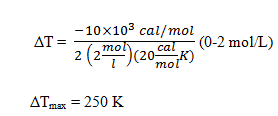
The adiabatic temperature rise indicates the potential danger of a coolant system failure. In this case the reactants contain enough internal energy to raise the reactor temperature by 250 K.
7. “All of the coolant is at a uniform temperature, Tc”, is this statement correct according to the assumptions for the Unsteady-state energy balance?
a) True
b) False
Explanation: All of the coolant is at a uniform temperature, Tc as the increase in coolant temperature as the coolant passes through the coil is neglected.
8. The evaporator economy is dependent on the ________
a) Heat transfer rate
b) Mass transfer rate
c) Energy balance considerations
d) Mass balance considerations
Explanation: There are three main measures of evaporator performance:
Capacity (kg vaporized/time), Economy (kg vaporized/ kg steam input), Steam consumption (kg/hr).
The performance of a evaporator is evaluated by the capacity and the economy.
The rate of heat transfer q through the heating surface of an evaporator, by the definition of overall heat transfer coefficient, is product of three factors
i) The area of heat transfer surface A
ii) The overall heat transfer coefficient U
iii) The overall temperature drop ΔT
Q = U * A * ΔT.
9. The fouling factor is _________
a) Is a safety factor
b) Accounts for all resistances due to mass transfer
c) Accounts for all resistances due to heat transfer
d) Accounts for all resistances due to heat transfer
Explanation: The fouling factor represents the theoretical resistance to heat flow due to a build-up of a layer of dirt or other fouling substance on the tube surfaces of the heat exchanger, but they are often overstated by the end user in an attempt to minimise the frequency of cleaning. In reality, if the wrong fouling factor is used, cleaning may actually be required more frequently.
10. The units for the log mean temperature difference are __________
a) 1 / °C
b) °C
c) 1/2 °C
d) Dimensionless
Explanation: In the particular system, whatever unit is assigned to temperature, the same is the unit of LMTD. It is just a special kind of temperature difference. Logarithmic mean temperature difference. LMTD is a logarithmic average of the temperature difference between the hot and cold streams in heat exchanger. Its unit is whatever is the unit of the temperature. °F or °C or Kelvin.