1. The growth of S.cerevisiae on glucose under anaerobic conditions can be described by the following reaction:
C6H12O6 + aNH3 → 0.59 CH1.64N0.16O0.52 (biomass) + 0.43 C3H8O3 + 1.54CO2 + 1.3C2H5OH +0.036H2O
Determine the biomass (MW= 23.74) yield coefficient Y x/s
a) 0.078 g. g⁻¹
b) 0.070 g. g⁻¹
c) 0.068 g. g⁻¹
d) 0.060 g. g⁻¹
Explanation: Yx/s = 0.59xMW biomass/1xMWglucose
= 0.59×23.74/1×180 = 0.078 g. g⁻¹.
2. The growth of S.cerevisiae on glucose under anaerobic conditions can be described by the following reaction:
C6H12O6 + aNH3 → 0.59 CH1.64N0.16O0.52 (biomass) + 0.43 C3H8O3 + 1.54CO2 + 1.3C2H5OH + 0.036H2O and determine the product yield coefficient Y etoh/s and determine coefficient a.
a) 0.57 g. g⁻¹, 0.078
b) 0.33 g. g⁻¹, 0.094
c) 0.45 g. g⁻¹, 0.086
d) 0.44 g. g⁻¹, 0.056
Explanation: Yetoh/s = 1.3 x MW EtOH / 1x MW glucose
= 0.59 x 46/ 1×180 = 0.33 g.g⁻¹
N in NH3 = N in biomass (CH1.64N0.16O0.52)
∴ a = (0.59)(0.16) = 0.094.
3. What is the degree of reduction of glucose?
a) 4
b) 3
c) 12
d) 24
Explanation: Glucose is C6H12O6. The degree of reduction = 6 x (+4) + 12 x (+1) + 6 x (-2) = 24.
4. What is the COD (chemical oxygen demand) of ethanol, expressed as g COD/g ethanol?
a) 1.30 g COD/g ethanol
b) 32 g COD/g ethanol
c) 2.24 g COD/g ethanol
d) 2.09 g COD/g ethanol
Explanation: Ethanol (C2H5OH) has a degree of reduction of 12 (mol electrons/mol ethanol). Oxygen has a degree of reduction of -4 (mol electrons/mol oxygen)and a molecular weight of 32 g/mol, which corresponds to 8 g oxygen (=COD)/mol electrons. Multiplying 12 by 8, this results in 96 g COD/mol ethanol, or, 96/46 = 2.09 g COD/g ethanol.
5. High energy input for downstream processing is maximized.
a) True
b) False
Explanation: Energy input for downstream processing is minimised to avoid damaging heat-labile products. Nevertheless, energy effects are important because biological catalysts are very sensitive to heat and changes in temperature. In large-scale processes, heat released during reaction can cause cell death or denaturation of enzymes if it is not quickly removed.
6. Which of the following is not an intensive property?
a) Temperature
b) Density
c) Mass
d) Mole fraction
Explanation: Temperature, density, and mole fraction are examples of properties which are independent of the size of the system; these quantities are called intensive variables. On the other hand, mass, volume and energy are extensive variables which change if mass is added to or removed from the system.
7. How much heat is produced by a human body?
a) 200 W
b) 100 W
c) 50 W
d) 0.5 W
Explanation: A man doing no or very little physical work needs about 2,000 kcal (or less) of energy in his daily food. The body converts this energy almost entirely into heat.
1 day = 24 x 60 x 60 s = 86,400 s 1 cal = 4.2 J
Hence, 2000 kcal/day = 2000 × 4.2 kj/day = \(\frac{8.4 \,Mj}{86600s}\)
= 100 j/s = 100 W
We see that a human body doing no work is equivalent to a heat source of about 100 W – the equivalent of a good bulb
8. 1 watt is equal to how much horsepower (hp) unit?
a) 0.001341 hp
b) 0.001241 hp
c) 0.001141 hp
d) 0.001151 hp
Explanation: 1 watt = 1 W = 1 J/1 s = 10-3 kW = 10-6 MW
= 3.412 BTU/h
= 0.001341 hp (horsepower units).
9. An automobile has a horsepower rating (power) of 100 hp. Calculate its power in watts.
a) 75.5 kW
b) 74.5 kW
c) 75.4 kW
d) 74.4 kW
Explanation:
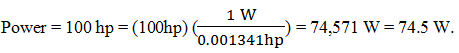
10. At what rate per hour does a 1 kW heater convert electrical energy into heat?
a) 3.5 MJ
b) 2.5 MJ
c) 3.6 MJ
d) 2.6 MJ
Explanation: Energy = power x time
1 kW is 1,000 watts
Since 1 watt is 1 joule per sec
1,000 watts is 1,000 joules per sec
In one hour there are 3,600 seconds
Substituting in the equation energy = 1,000 x 3,600 joules
= 3,600,000 joules
= 3.6 MJ
Therefore a 1 kW heater converts 3.6 MJ of electrical energy into heat per hour.