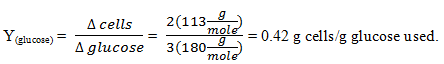1. What is the fractional and percent excess of H2 from the following equation?
H2 + Br2 = 2HBr
H2 = 25 mol/hr
Br2 = 20 mol/hr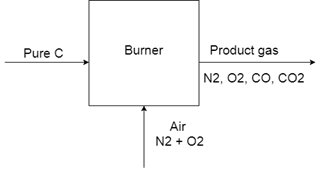
a) 0.45, 45%
b) 0.25, 25%
c) 0.75, 75%
d) 0.65, 65%
Explanation: Fractional excess H2 = (25-20)/ 20 = 0.25
Percent excess = 25%.
2. When pure carbon is burned in air, some of it oxidizes into CO2 and some to CO. The molar ratio of N2 to O2 is 7.18 and the ratio of CO to CO2 is 2.0 in the product gas. What is the percent excess air used? The exit gases contain only N2, O2, CO and CO2.
a) 50 %
b) 60 %
c) 40 %
d) 20 %
Explanation: The process involves two reactions:
R1 = C + O2 = CO2
R2 = C + 1/2 O2 = CO
As the product (output) gas composition is specified more clearly, we may use it as starting point.
Take N2 in the product as nN2 = Take N2 in the product as, then O2 = 1 mol
N2 as an inert gas: input = output = 7.18 mol
O2 input with air = 7.18 (21/79) = 1.91 mol
Total O2 consumption = 1.91-1 = 0.91 mol
If R1 uses n1 mol O2, generating n1 mol CO2, R2 uses 0.91-n1 mol O2, generating 2
(0.91-n1) mol CO.
Since CO/CO2 in the product gas = 2, 2 (0.91-n1)/n1 = 2
n1 = 0.91/2 = 0.455 mol
Therefore, total moles of C input = 3 n1 = 1.365 mol
O2 required for complete combustion of 1.365 mol C = 1.365 mol (for R1 only)
O2 excess % = (1.91 -1365)/1.365 x 100% = 39.9% = 40% .
3. Convert 800 mmHg into bars.
a) 1.064 bars
b) 1.066 bars
c) 1.054 bars
d) 1.055 bars
Explanation: 1 mm Hg = 133.322 Pa and 1 bar = 105 Pa
Therefore,
bars = 800 mm Hg × \(\frac{133.322 \,Pa}{1 \,mm \,Hg} × \frac{1 \,bar}{10^5 \,Pa}\) = 1.066 bar.
4. What do you mean by material balance equation?
a) Original-Solids-in-place (OSIP)
b) Original-Gas-in-place (OGIP)
c) Original-Air-in-place (OAIP)
d) Original-Liquid-in-place (OLIP)
Explanation: Material balance analysis is an interpretation method used to determine original fluids-in-place (OFIP) based on production and static pressure data. The general material balance equation relates the original oil, gas, and water in the reservoir to production volumes and current pressure conditions / fluid properties.
5. ”Bacteria have slightly higher nitrogen content than fungi”.
a) True
b) False
Explanation: Bacteria tend to have slightly higher nitrogen contents (11-14%) than fungi (6.3-9.0%).
6. Estimate the degree of reduction of Methane, Glucose and Ethanol?
a) γ(CH4) = 6, γ(C6H12O6) = 4, γ(C2H5OH) = 6
b) γ(CH4) = 6, γ(C6H12O6) = 4, γ(C2H5OH) = 8
c) γ(CH4) = 8, γ(C6H12O6) = 4, γ(C2H5OH) = 6
d) γ(CH4) = 4, γ(C6H12O6) = 6, γ(C2H5OH) = 8
Explanation:
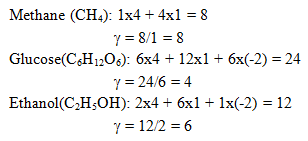
7. Calculate the stoichiometric coefficients of the following biological reaction:
Hexadecane: C16H34 + a O2 + b NH3 = c (C4.4H7.3N0.86O1.2) + d H2O + e CO2
a) a = 12.427, b = 2.085, c = 2.42, d = 12.43, e = 5.33
b) a = 12.345, b = 3.456, c = 2.42, d = 12.46, e = 5.44
c) a = 12.594, b = 2.345, c = 3.42, d = 12.49, e = 5.66
d) a = 12.345, b = 3.560, c = 3.42, d = 12.46, e = 5.44
Explanation: Amount of carbon in 1 mole of substrate = 16 (12) = 192 g
Amount of carbon converted to biomass = 192 (2/3) = 128 g
Then, 128 = c (4.4)(12); c = 2.42
Amount of carbon converted to CO2 = 192 – 128 = 64 g
64 = e (12)
e = 5.33
The nitrogen balance yields
14b = c (0.86)(14)
b = (2.42)(0.86) = 2.085
The hydrogen balance is
34 (1) + 3b = 7.3c + 2d
d = 12.43
The oxygen balance yields
2a(16) = 1.2c(16) + 2e(16) + d(16)
a = 12.427.
8. Calculate the stoichiometric coefficients of the following biological reaction:
Glucose: C6H12O6 + aO2 + bNH3 = c (C4.4H7.3N0.86O1.2) + d H2O + e CO2
a) a = 1.573, b = 0.685, c = 0.470, d = 2.564, e = 2
b) a = 2.789, b = 1.896, c = 0.438, d = 1.395, e = 1
c) a = 1.473, b = 0.782, c = 0.909, d = 3.854, e = 2
d) a = 2.390, b = 1.295, c = 0.943, d = 2. 564, e = 1
Explanation: Amount of carbon in 1 mole of substrate = 72 g
Amount of carbon converted to biomass = 72(2/3) = 48 g
Then, 48 = 4.4c (12); c= 0.909
Amount of carbon to CO2 = 72-48 = 24 g
24 = 12e
e = 2
The nitrogen balance yields
14b = 0.86c (14)
b = 0.782
The hydrogen balance is
12 + 3b = 7.3c + 2d
d = 3.854
The oxygen balance yields
6(16) + 2(16)a = 1.2(16)c + 2(16)e +16d
a = 1.473.
9. A yeast (CH1.66N0.13O0.40) is growing aerobically on arabinose (C5H10O5) and ammonium hydroxide (NH4OH) with a respiratory quotient of 1.4. Estimate the stoichiometric coefficient of the equation:
aC5H10O5 + bO2 + cNH4OH → CH1.66N0.13O0.40 + dCO2 + eH2O
a) a = 0.2823, b = 0.2938, c = 0.13, d = 0.4113, e = 0.9065
b) a = 0.2893, b = 0.3638, c = 0.13, d = 0.4321, e = 0.9478
c) a = 0.2843, b = 0.2590, c = 0.13, d = 0.4321, e = 0.9576
d) a = 0.2823, b = 0.2130, c = 0.13, d = 0.4113, e = 0.8743
Explanation: Equations for coefficients:
C atom balance: 5a = 1 + d —————— (1)
H atom balance: 10a + 5c = 1.66 + 2e ——– (2)
O atom balance: 5a + 2b + c = 0.40 + 2d + e —- (3)
N atom balance: c = 0.13 ———————— (4)
Respiratory quotient: RQ = 1.4 = d/b ———– (5)
Therefore, eq.2× eq.(3) – eq.(2), we get,
b = 0.2938
From eq.(5), we get,
d = 0.4113
From eq.(1), we get,
a = 0.2823
From eq.(3), we get,
e = 0.9065.
10. Estimating Yield from Stoichiometry:
3C6H12O6 + 8 O2 + 2NH3 -> 2C5H7O2N + 8CO2 +14H2O
Given:3(180) 8(32) 2(17) 2(113)
Calculate the yield of glucose.
a) 0.53 g cells / g glucose used
b) 0.27 g cells / g glucose used
c) 0.42 g cells / g glucose used
d) 0.51 g cells / g glucose used
Explanation: