1. Which of the following processes does not involve a catalyst?
a) Thermite process
b) Ostwald process
c) Contact process
d) Haber process
Explanation: In thermite process no catalyst is required
2. The factor responsible for weak acidic nature of B–F bonds
in \[BF_{3}\] is
a) large electronegativity of fluorine
b) three centred two electron bonds in \[BF_{3}\]
c) \[p\pi-d\pi\] back bonding
d) \[p\pi-p\pi\] back bonding
Explanation:

3. An aqueous solution of \[FeSO_{4}, Al_{2}(SO_{4})_{3}\] and chrome alum
is heated with excess of \[Na_{2}O_{2}\] and filtered. The materials
obtained are :
a) a colourless filtrate and a green residue
b) a yellow filtrate and a green residue
c) a yellow filtrate and a brown residue
d) a green filtrate and a brown residue
Explanation:

4. Which of the following has the minimum heat of dissociation:
a) \[\left(CH_{3}\right)_{3}N:\rightarrow BF_{3}\]
b) \[\left(CH_{3}\right)_{3}N:\rightarrow B\left(CH_{3}\right)_{2}F\]
c) \[\left(CH_{3}\right)_{3}N:\rightarrow B\left(CH_{3}\right)_{3}\]
d) \[\left(CH_{3}\right)_{3}N:\rightarrow B\left(CH_{3}\right)F_{2}\]
Explanation: Due to + I effect of methyl groups the Lewis character of B(CH3)3 decreases and coordination becomes weaker.
5. Purification of alumina is essential because :
a) impure alumina has a very high melting point
b) impure alumina is a very poor conductor of electricity
c) impure alumina cannot react with the oxidizing agent
d) it is difficult to purify aluminium metal
Explanation: Statement (d) is correct.
6. Which reaction cannot give anhydrous \[AlCl_{3}\] :
a) Passing dry \[Cl_{2}\] over heated aluminium powder.
b) Heating a mixture of alumina and coke in a current of dry
\[Cl_{2}\].
c) Passing dry HCl over heated aluminium powder
d) Heating of \[AlCl_{3}.6H_{2}O\]
Explanation:
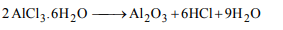
7. The process used for purification of bauxite ore containing
iron oxide impurity is known as :
a) Hoope’s process
b) Serpeck’s process
c) Baeyer’s process
d) Electrolytic process
Explanation: Bauxite ore containing Fe2O3 is purified by Baeyer’s process.
8. Which statement is not true about potash alum ?
a) On heating it melts and loses its water of crystallization
b) It’s aqueous solution is basic in nature.
c) It is used in dyeing industries.
d) It’s empirical formula is \[KAl\left(SO_{4}\right)_{2}.12H_{2}O\]
Explanation: Alum form acidic solution due to hydrolysis of Al3+
9. Which statement is false
a) Water gas is a mixture of hydrogen and carbon dioxide.
b) Producer gas is a mixture of CO and Nitrogen
c) Water gas is a mixture of water vapour and hydrogen
d) Natural gas consists of methane, ethane and gaseous
hydrocarbons.
Explanation: "Water gas is a mixture of hydrogen and carbon dioxide". This is False.
10. Silicon has a strong tendency to form polymers like silicones.
The chain length of silicone polymer can be controlled by
adding
a) \[MeSiCl_{3}\]
b) \[Me_{2}SiCl_{2}\]
c) \[Me_{3}SiCl\]
d) \[Me_{4}Si\]
Explanation: \[Me_{3}SiCl\]