1. The dissolution of \[Al\left(OH\right)_{3}\] by a solution of NaOH results in
the formation of
a) \[\left[Al\left(H_{2}O\right)_{4}\left(OH\right)\right]^{2+}\]
b) \[\left[Al\left(H_{2}O\right)_{2}\left(OH_{4}\right)\right]^{-}\]
c) \[\left[Al\left(H_{2}O\right)_{3}\left(OH\right)_{3}\right]\]
d) \[\left[Al\left(H_{2}O\right)_{6}\left(OH\right)_{3}\right]\]
Explanation:
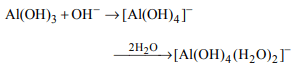
2. Which of the following is pseudo alum ?
a) \[\left(NH_{4}\right) _{2}SO_{4}.Fe_{2} \left(SO_{4}\right)_{3}.24H_{2}O\]
b) \[K _{2}SO_{4}.Al_{2} \left(SO_{4}\right)_{3}.24H_{2}O\]
c) \[MnSO_{4}.Al_{2} \left(SO_{4}\right)_{3}.24H_{2}O\]
d) None of these
Explanation:

3.Which of the following statements about anhydrous
aluminium chloride is correct ?
a) It exist as \[AlCl_{3}\] molecule
b) It is a strong lewis base
c) It sublimes at 100°C under vaccum
d) It is not easily hydrolysed
Explanation: AlCl3 is Lewis acid, exists as dimer (Al2 Cl6 ) and easily hydrolysed
4. The two type of bonds present in \[B_{2}H_{6}\] are covalent and
a) ionic
b) co-ordinate
c) hydrogen bridge bond
d) None of these
Explanation: B2H6 contains hydrogen bridge bonds. These are one electron bonds also known as banana bonds
5. Orthoboric acid when heated to red hot gives
a) metaboric acid
b) pyroboric acid
c) boron and water
d) boric anhydride
Explanation:
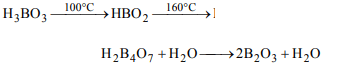
6. Anodised aluminium is
a) Al obtained at anode
b) Al prepared electrolytically
c) Alloy of Al containing 95% of Al
d) Al electrolytially coated with aluminium oxide
Explanation: Al electrolytically coated with aluminium oxide is known as anodised aluminium
7. Which statement regarding \[H_{3}BO_{3}\] is not correct ?
a) It is a strong tribasic acid
b) It is prepared by acidifying an aqueous solution of borax
c) It has a layer structure in which planar \[BO_{3}\] units are
joined by H- bonds
d) It does not act as proton donor but acts on lewis acid by
accepting OH– ions
Explanation:
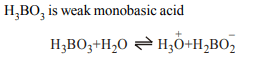
8. The precious Ruby stone is
a) alumina
b) aluminium silicate
c) sodium aluminium silicate
d) sodium silicate
Explanation: Alumina Al2O3 is known as Ruby stone
9.The hybridisation of boron atom in orthoboric acid is
a) sp
b) \[sp^{2}\]
c) \[sp^{3}\]
d) \[sp^{3}d\]
Explanation: The hybridizations of B in H3 BO3 is sp2
10. Which of the following statements is not correct ?
a) Al acts as a reducing agent
b) Al does not react with steam even at higher temperature
c) Al forms a number of alloys with other metals
d) Al is ionic in all its compounds
Explanation: Al in its compounds forms covalent bonds.