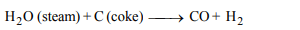1. Which of the folllowing is not correct?
a) \[Ge \left(OH\right)_{2}\] is amphoteric
b) \[GeCl_{2}\] is more stable than \[GeCl_{4}\]
c) \[GeO_{2}\] is weakly acidic
d) \[GeCl_{4}\] in HCl forms \[\left[GeCl_{2}\right]^{2-}\] ion
Explanation: Ge4+ is more stable than Ge2+ . Hence GeCl4 is more stable than GeCl2
2.Producer gas, a fuel and also a source of nitrogen is obtained
by
a) passing a mixture of steam and air over incandescent
coke.
b) spraying oil into hot retorts
c) restricted supply of air through a bed of incandescent
coke
d) passing steam over incandescent coke.
Explanation: Producer gas is mixture of CO + N2 . It is prepared by incomplete combustion of coal in limited supply of air
3.Which of the following shows bond in silicone :
a) Si – Si – Si – Si
b) – Si – O – Si – O – Si
c) Si – C – Si – C – Si
d) Si – C – Si – O – Si
Explanation:
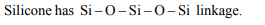
4. Which type of forces bind together the carbon atoms in
diamond ?
a) Coulombic forces
b) van der Waal’s forces
c) Dipole-Dipole forces
d) Covalent forces
Explanation: In diamond each carbon is sp3 hybridised and linked to other C-atoms by covalent bonds.
5.Which is the best absorbing material for carbon dioxide?
a) Cold, solid calcium chloride
b) Heated charcoal
c) Heated copper oxide
d) Cold, solid calcium hydroxide
Explanation: CO2 is acidic in nature and it require most basic substance for absorption.
6. Water gas cannot be prepared by a continuous process
because
a) the reaction ceases when coke is too cool
b) it cannot be manufactured without producer gas
c) the furnace must be allowed to cool occasionally
d) hot coke must be added from time to time.
Explanation:
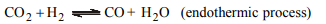
7. A kettle which becomes furred-up in use has inside it a deposit
composed mainly of
a) magnesium bicarbonate
b) magnesium sulphate
c) sodium sulphate
d) calcium carbonate
Explanation: The bicarbonates of Ca and Mg are decomposed to Ca and Mg carbonates by boiling.
8. The substance used to impart green colour to glass is :
a) \[Cu_{2}O\]
b) \[SnO_{2}\]
c) \[Cr_{2}O_{3}\]
d) CdS
Explanation: Cr2O3 impart green colour to glass.
9. On doping Ge metal with a little of In, one gets :
a) insulator
b) rectifier
c) n-type semiconductor
d) p-type semiconductor
Explanation: Doping of Ge with In produces p-type semiconductor
10. Water gas is produced by :
a) saturating hydrogen with moisture
b) passing steam through a red hot coke bed.
c) mixing oxygen and hydrogen in the ratio of 1 : 2
d) heating a mixture of \[CO_{2}\] and \[CH_{4}\] in petroleum
refineries
Explanation: