1. When sodium oxide is heated in a current of CO2 at 360°C we
get
a) sodium formate
b) sodium oxalate
c) sodium acetate
d) sodium carbonate
Explanation:
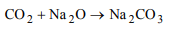
2. Tin plague is the
a) conversion of stannous to stannic
b) conversion of white tin to grey tin
c) emission of sound while bending a tin rod
d) atmospheric oxidation of tin
Explanation: It is the conversion of white tin to grey tin at low temperature which crumbles into powder.
3. A gas does not turn lime water milky, supports combustion
of burning magnesium. It has no smell and is colourless. It
extinguishes a glowing splint but under some circumstances
reacts with oxygen and hydrogen. It is not poisonous. The
gas is likely to be
a) water vapours
b) nitrogen
c) \[CO_{2}\]
d) helium
Explanation: All mentioned properties are for nitrogen
4. The correct statement with respect to CO is
a) it combines with H2O to give carbonic acid
b) it reacts with haemoglobin in RBC
c) it is powerful oxidising agent
d) it is used to prepare aerated drinks
Explanation: CO react with haemoglobin, forms carboxy haemoglobin and stopes the supply of O2
5. Tin cry refers to
a) conversion of white to grey tin
b) tin plating
c) conversion of white tetrahedral tin to white rhombohedral
tin
d) emission of sound while bending a tin rod.
Explanation: Tin cry is the emission of sound on bending the tin
6. Plumbo - solvency means dissolution of lead in
a) hot water
b) acids
c) ordinary water
d) alkalies
Explanation:
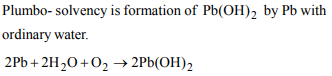
7.The reducing power of divalent species decreases in the
order
a) Ge > Sn > Pb
b) Sn > Ge > Pb
c) Pb > Sn > Ge
d)None of these
Explanation:
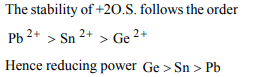
8. Which is formed when \[SiCl_{4}\] vapours are passed over
hot Mg
a) \[SiCl_{2}+MgCl_{2}\]
b) \[Si+MgCl_{2}\]
c) \[Mg_{2}Si+Cl_{2}\]
d) \[MgSiCl_{6}\]
Explanation:
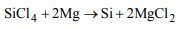
9. \[Mg_{2}C_{3}\] possess which of the following characteristics?
a) Is called magnesium allylide
b) It contain \[Mg^{2+}\] and \[C_3^ {4-}\] ions
c) It on hydrolysis gives propyne
d) All of these
Explanation:
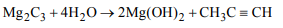
10. Lead is not affected by dil. HCl in cold because
a) Pb is less electronegative than H
b) PbO film is formed which resists chemical attack by acid
c) \[PbCl_{2}\] protective coating gets formed on Pb surface
d) \[PbO_{2}\] film is always present on Pb surface, which resist
chemical attack
Explanation: Pb with dil HCl forms protective coating of PbCl2