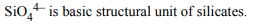1. The ion that can be precipitated by HCl as well as \[H_{2} S\] is
a) \[Pb^{2+}\]
b) \[Fe^{3+}\]
c) \[Zn^{2+}\]
d) \[Cu^{2+}\]
Explanation: Pb2+ can be precipitated as PbCl2 in cold water and as PbS in II group by H2 S in acid medium
2. Which of the following is most dense?
a) Fe
b) Cu
c) B
d) Pb
Explanation: Pb is most dense by virtue of its property
3. C and Si have
a) Same physical properties
b) Different physical properties
c) Same physical but different chemical properties
d) Different chemical and physical properties
Explanation: C and Si have different physical and chemical properties
4. Mark the oxide which is amphoteric in character
a) \[CO_{2} \]
b) \[SiO_{2} \]
c) \[SnO_{2} \]
d) CaO
Explanation: CO2 , SiO2 are acidic, CaO is basic and SnO2 is amphoteric.
5. Which of the following sulphate is insoluble in water?
a) \[CuSO_{4} \]
b) \[CdSO_{4} \]
c) \[PbSO_{4} \]
d) \[Al_{2}\left(SO _{4}\right)_{3}\]
Explanation: PbSO4 is insoluble in water
6. Which of the following molecule has highest bond energy?
a) F-F
b) C-C
c) N-N
d) O-O
Explanation:
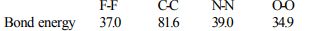
7. The metal used for making radiation shield is
a) Al
b) Fe
c) Zn
d) Pb
Explanation: Since lead can stop harmful radiation. Hence it is used for making radiation shield.
8. The straight chain polymer is formed by:
a) hydrolysis of \[CH_{3}SiCl_{3}\] followed by condensation
polymerisation
b) hydrolysis of \[\left(CH_{3}\right)_{4}Si\] by addition polymerisation
c) hydrolysis of \[\left(CH_{3}\right)_{2}SiCl_{2}\] followed by condensation
polymerisation
d) hydrolysis of \[\left(CH_{3}\right)_{3}SiCl\] followed by condensation
polymerisation
Explanation:
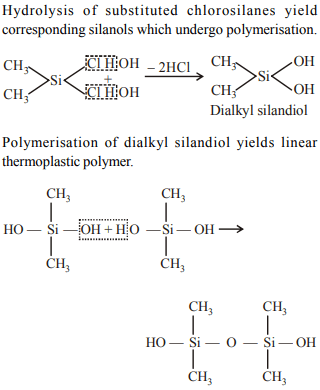
9. Name the type of the structure of silicate in which one oxygen
2tom of \[\left[SiO_{4}\right]^{4-}\] is shared ?
a) Linear chain silicate
b) Sheet silicate
c) Pyrosilicate
d) Three dimensional
Explanation:
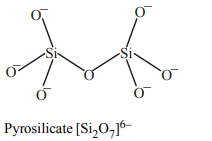
10. The basic structural unit of silicates is :
a) \[SiO_{4}^{4-}\]
b) \[SiO_{3}^{2-}\]
c) \[SiO_{4}^{2-}\]
d) SiO
Explanation: