1. The stability of the following alkali metal chlorides follows the order
a) LiCl > KCl > NaCl > CsCl
b) CsCl > KCl > NaCl > LiCl
c) NaCl > KCl > LiCl > CsCl
d) KCl > CsCl > NaCl > LiCl
Explanation:
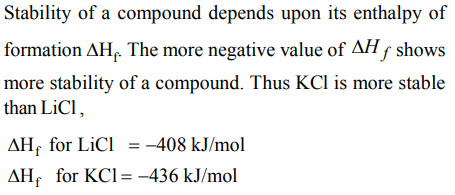
2. The order of solubility of lithium halides in non polar solvents follows the order :
a) LiI > LiBr > LiCl > LiF
b) LiF > LiI > LiBr > LiCl
c) LiCl > LiF > LiI > LiBr
d) LiBr > LiCl > LiF > LiI
Explanation: As the size of the anion increases from F– to I– , the covalent character increase and hence the solubility in non -polar solvent increases LiI > LiBr > LiCl > LiF
3. Which among the following is most soluble in water
a) \[ CsClO_{4}\]
b) \[ NaClO_{4}\]
c) \[ LiClO_{4}\]
d) \[ KClO_{4}\]
Explanation: The high solubility of LiClO4 is mainly due to high heat of hydration of Li+ ion
4. When sodium is treated with sufficient oxygen/air the product obtained is
a) \[ Na_{2}O\]
b) \[ Na_{2} O_{2}\]
c) \[ NaO_{2}\]
d) NaO
Explanation: Na in excess of O2 forms Na2O2
5. Choose the compound which does not possess a peroxide group
a) \[ Na_{2} O_{2}\]
b) \[CrO_{5}\]
c) \[ Fe_{2} O_{3}\]
d) \[BaO_{2}\]
Explanation:
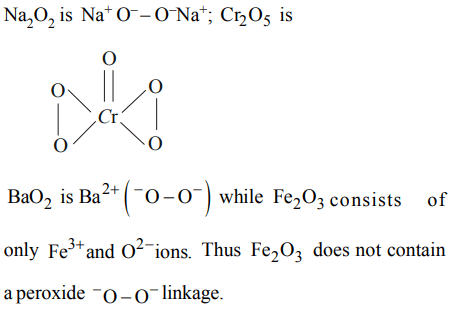
6. Which of the following alkali metals burns in air to form only monoxide ?
a) Na
b) Li
c) K
d) Cs
Explanation: Li
7. Alkali metals form peroxides and superoxides except
a) Na
b) Rb
c) Li
d) Cs
Explanation: Li+ does not allow its O2- ion to combine with other O atom(s) to form peroxides and superoxides
8. Which is the most basic of the following?
a) \[Na_{2}O\]
b) BaO
c) \[ As_{2}O_{3}\]
d) \[ Al_{2}O_{3}\]
Explanation: \[Na_{2}O\]
9. Which of the following is used as a source of oxygen in space capsules, submarines and breathing masks ?
a) \[Li_{2}O\]
b) \[Na_{2}O_{2}\]
c) \[KO_{2}\]
d) \[K_{2}O_{2}\]
Explanation:
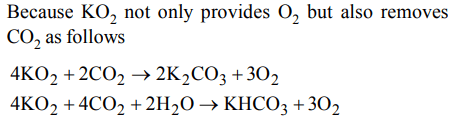
10. Which of the following oxides of potassium is not known ?
a) \[K_{2}O\]
b) \[K_{2}O_{4}\]
c) \[KO_{3}\]
d) \[K_{2}O_{3}\]
Explanation: O42- ion is not possible and K2O4 is unknown