1. Which of the following has lowest thermal stability ?
a) \[Li_{2}CO_{3}\]
b) \[Na_{2}CO_{3}\]
c) \[K_{2}CO_{3}\]
d) \[Rb_{2}CO_{3}\]
Explanation: The weaker the base, the less stable is its carbonate. Since LiOH is the weakest base, hence Li2CO3 has the lowest thermal stability.
2. Which one of these is basic ?
a) \[CO_{2}\]
b) \[SiO_{2}\]
c) \[Na_{2}O\]
d) \[SO_{2}\]
Explanation: \[Na_{2}O\]
3. Sodium nitrate decomposes above 800°C to give
a) \[N_{2}\]
b) \[O_{2}\]
c) \[NO_{2}\]
d) \[Na_{2}O\]
Explanation:
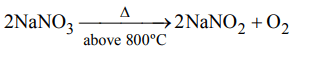
4. Fires , that result from the combustion of alkali metals can be extinguished by
a) \[CCl_{4}\]
b) sand
c) water
d) kerosene
Explanation: In contrast, CCl4 is a low boiling highly non inflammable heavy liquid. Its vapours surround the fire thereby cutting off air supply
5. The alkali metal that reacts with nitrogen directly to from nitride is
a) Li
b) Na
c) K
d) Rb
Explanation: Li
6. Which of the following bicarbonates does not exist as solid?
a) \[KHCO_{3}\]
b) \[NaHCO_{3}\]
c) \[CsHCO_{3}\]
d) \[LiHCO_{3}\]
Explanation: LiHCO3 is unstable and exists only in solution .
7. Which of the following is most stable?
a) \[Na_{3}N\]
b) \[Li_{3}N\]
c) \[K_{3}N\]
d) \[Rb_{3}N\]
Explanation: Only Li3N is stable ,others are not formed at all .
8. \[ LiNO_{3}\] on heating gives
a) \[ O_{2}\]
b) \[ NO_{2}\]
c) \[ O_{2}+ NO_{2}\]
d) None of these
Explanation:
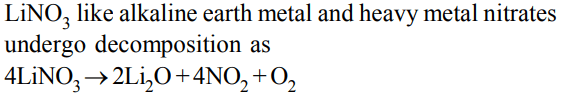
9. Which of the following does not form an oxide on heating ?
a) \[ ZnCO_{3}\]
b) \[ CaCO_{3}\]
c) \[ Li_{2} CO_{3}\]
d) \[ Na_{2} CO_{3}\]
Explanation: Na2CO3 does not decompose to form Na2O
10. Alkali metal hydrides react with \[ H_{2}O\] to give
a) basic solution
b) acidic solution
c) neutral solution
d) hydrogen gas
Explanation: hydrogen gas