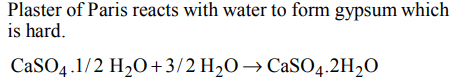1. Property of the alkaline earth metals that increases with their atomic number is
a) solubility of their hydroxides in water
b) solubility of their sulphates in water
c) ionization energy
d) electronegativity
Explanation: Lattice energy decreases more rapidly than hydration energy for alkaline earth metal hydroxides. On moving down a group solubility of their hydroxides increases
2. The compound A on heating gives a colourless gas and aresidue that is dissolved in water to obtain B. Excess of \[CO_{2}\] is bubbled through aqueous solution of B, C is formed which
is recovered in the solid form. Solid C on gentle heating gives back A. The compound is
a) \[CaSO_{4}.2H_{2}O\]
b) \[CaCO_{3}\]
c) \[Na_{2}CO_{3}\]
d) \[K_{2}CO_{3}\]
Explanation:
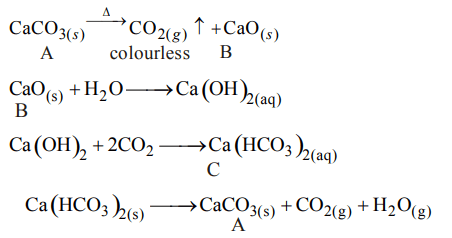
3. Which of the following compounds has the lowest melting point ?
a) \[CaCl_{2}\]
b) \[CaBr_{2}\]
c) \[CaI_{2}\]
d) \[CaF_{2}\]
Explanation: Melting points of halides decreases as the size of the halogen increases. The correct order is CaF2 > CaCl2 > CaBr2 > CaI2
4. Which one of the following is present as an active ingredient in bleaching powder for bleaching action ?
a) \[CaOCl_{2}\]
b) \[Ca\left(OCl\right)_{2}\]
c) \[CaO_{2}Cl\]
d) \[CaCl_{2}\]
Explanation: Active ingredient in bleaching powder for bleaching action is Ca(OCl)2
5. Equimolar solutions of the following substances were prepared separately. Which one of these will record the highest pH value ?
a) \[BaCl_{2}\]
b) \[AlCl_{3}\]
c) LiCl
d) \[BeCl_{2}\]
Explanation:

6. A metal M readily forms its sulphate \[MSO_{4}\] which is watersoluble. It forms its oxide MO which becomes inert on heating. It forms an insoluble hyroxide \[M\left(OH\right)_{2}\] which is soluble in
NaOH solution. Then M is
a) Mg
b) Ba
c) Ca
d) Be
Explanation: Beryllium shows anomalous properties due to its small size.
7. The solubilities of carbonates decrease down the magnesium
group due to a decrease in
a) hydration energies of cations
b) inter-ionic attraction
c) entropy of solution formation
d) lattice energies of solids
Explanation:

8. In curing cement plasters water is sprinkled from time to time. This helps in
a) developing interlocking needle-like crystals of hydrated silicates
b) hydrating sand and gravel mixed with cement
c) converting sand into silicic acid
d) keeping it cool
Explanation: Setting of cement is exothermic process which develops interlocking crystals of hydrated silicates
9. Which of the following on thermal decomposition yields a basic as well as acidic oxide ?
a) \[NaNO_{3}\]
b) \[KClO_{3}\]
c) \[CaCO_{3}\]
d) \[NH_{4}NO_{3}\]
Explanation:
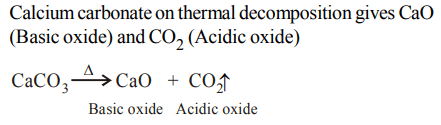
10. Plaster of Paris hardens by
a) giving off \[CO_{2}\]
b) changing into \[CaCO_{3}\]
c) uniting with water
d) giving out water
Explanation: