1. The enthalpy of fusion of water is 1.435 kcal/mol. The molar entropy change for the melting of ice at 0°C is :
a) 10.52 cal / (mol K)
b) 21.04 cal / (mol K)
c) 5.260 cal / (mol K)
d) 0.526 cal / (mol K)
Explanation:
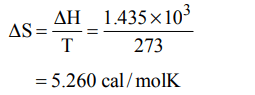
2. Standard enthalpy of vapourisation \[\triangle_{vap}H^{\circ}\] for water at 100°C is \[40.66 k J mol^{-1}\] . The internal energy of vaporisation
of water at \[100°C\left(kJ mol^{-1}\right)\] is :
a) + 37.56
b) – 43.76
c) + 43.76
d) + 40.66
Explanation:
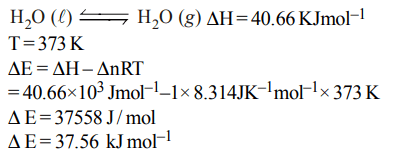
3. Equal volumes of two monoatomic gases, A and B, at same temperature and pressure are mixed. The ratio of specific heats \[\left(C_{P}\diagup C_{V}\right)\] of the mixture will be :
a) 0.83
b) 1.50
c) 3.3
d) 1.67
Explanation:
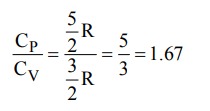
4. A reaction having equal energies of activation for forward and reverse reaction has :
a) \[\triangle G=0\]
b) \[\triangle H=0\]
c) \[\triangle H=\triangle G=\triangle S=0\]
d) \[\triangle S=0\]
Explanation:
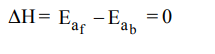
5. If an endothermic reaction is non-spontaneous at freezing point of water and becomes feasible at its boiling point, then
a) \[\triangle H \] is –ve, \[\triangle S \] is +ve
b) \[\triangle H\] and \[\triangle S\] both are +ve
c) \[\triangle H\] and \[\triangle S\] both are –ve
d) \[\triangle H \] is +ve, \[\triangle S \] is -ve
Explanation: At low temperature the \[\triangle\]S is – ve which makes \[\triangle\]G positive (\[\triangle\]G = \[\triangle\]H – T\[\triangle\]S). But at higher temperature \[\triangle\]S is +ve which makes the \[\triangle\]G negative (condition for spontaneity)
6. A heat engine abosrbs heat \[Q_{1}\] at temperature \[T_{1}\]and heat \[Q_{2}\] at temperature \[T_{2}\]. Work done by the engine is \[J\left(Q_{1}+Q_{2}\right)\] . This data
a) violates 1st law of thermodynamics
b) violates 1st law of themodynamics if \[Q_{1}\] is –ve
c) violates 1st law of thermodynamics of \[Q_{2}\] is –ve
d) does not violate 1st law of themodynamics
Explanation: It violate 2nd law of thermodynamics, not 1st
7. For the reactions,
\[C+O_{2}\rightarrow CO_{2};\triangle H= -393 J\]
\[2Zn+O_{2}\rightarrow 2 ZnO;\triangle H= -412 J\]
a) carbon can oxidise Zn
b) oxidation of carbon is not feasible
c) oxidation of Zn is not feasible
d) Zn can oxidise carbon
Explanation: \[\triangle\]H negative shows that the reaction is spontaneous. Higher value for Zn shows that the reaction is more feasible.
8. The internal energy change when a system goes from state
A to B is 40 kJ/mole. If the system goes from A to B by a
reversible path and returns to state A by an irreversible path
what would be the net change in internal energy ?
a) > 40 kJ
b) < 40 kJ
c) Zero
d) 40 kJ
Explanation: For a cyclic process the net change in the internal energy is zero because the change in internal energy does not depend on the path.
9. If at 298 K the bond energies of C — H, C — C, C = C and H — H bonds are respectively 414, 347, 615 and \[435 kJ mol^{-1}\] , the value of enthalpy change for the reaction
\[H_{2}C=CH_{2}\left(g\right)+H_{2}\left(g\right)\rightarrow H_{3}C-CH_{3}\left(g\right)\]
at 298 K will be
a) – 250 kJ
b) + 125 kJ
c) – 125 kJ
d) + 250 kJ
Explanation:
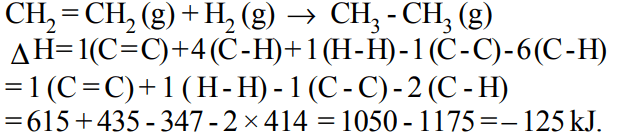
10. In an irreversible process taking place at constant T and P and in which only pressure-volume work is being done, the change in Gibbs free energy (dG) and change in entropy (dS), satisfy the criteria
a) \[\left(\triangle S\right)_{V,E}>0,\left(\triangle G\right)_{T,P}<0\]
b) \[\left(\triangle S\right)_{V,E}=0,\left(\triangle G\right)_{T,P}=0\]
c) \[\left(\triangle S\right)_{V,E}=0,\left(\triangle G\right)_{T,P}>0\]
d) \[\left(\triangle S\right)_{V,E}<0,\left(\triangle G\right)_{T,P}<0\]
Explanation: For spontaneous reaction, dS > 0 and \[\triangle\]G and \[\triangle\]G should be negative i.e. < 0