1. Enthalpy of a reaction \[\triangle H\] is expressed as
a) \[\triangle H=\sum\triangle H_{p}-\sum\triangle H_{R}\]
b) \[\triangle H=\triangle H_{p}+\triangle H_{R}\]
c) \[\triangle H=\frac{\triangle H_{p}}{\triangle H_{R}}\]
d) \[\triangle H=\frac{\sum H_{p}}{\sum H_{R}}\]
Explanation:
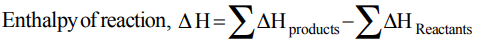
2. The enthalpy change of a reaction does not depend on
a) The state of reactants and products
b) Nature of reactants and products
c) Different intermediate reactions
d) Initial and final enthalpy change of a reaction
Explanation: In accordance with Hess’s law
3. Enthalpy change \[\left(\triangle H\right)\] of a system depends upon its
a) Initial state
b) Final state
c) Both on initial and final state
d) None of these
Explanation: Both on initial and final state
4. The relationship between enthalpy change and internal
energy change is
a) \[\triangle H=\triangle E+P\triangle V\]
b) \[\triangle H=\left(\triangle E+V\triangle P\right) \]
c) \[\triangle H=\triangle E-P\triangle V\]
d) \[\triangle H=V\triangle P -\triangle E\]
Explanation: \[\triangle\] H = \[\triangle\] E + P \[\triangle\] V
5. If a reaction involves only solids and liquids which of the following is true ?
a) \[\triangle H<\triangle E\]
b) \[\triangle H=\triangle E\]
c) \[\triangle H>\triangle E\]
d) \[\triangle H=\triangle E+RT\triangle n\]
Explanation: \[\triangle\] H = \[\triangle\] E + P \[\triangle\] V, for solid and liquid, \[\triangle\] V = 0 or \[\triangle\] H = \[\triangle\] E + \[\triangle\] nRT, for solids and liquids \[\triangle\] n = 0
6. \[C\left(diamond\right)\rightarrow C\left(graphite\right),\triangle H\] = -ve. This shows that
a) Graphite is more stable
b) Graphite has more energy than diamond
c) Both are equally stable
d) Stability cannot be predicted
Explanation:

7. One mole of a non-ideal gas undergoes a change of state \[\left(2.0 atm, 3.0 L, 95 K\right)\rightarrow\left(4.0 atm, 5.0 L, 245 K\right)\] with a change
in internal energy, \[\triangle U= 30.0 L atm\] . The change in enthalpy \[\triangle H\] of the process in L atm is.
a) 40.0
b) 42.3
c) 44.0
d) Not defined because pressure is not constant
Explanation:
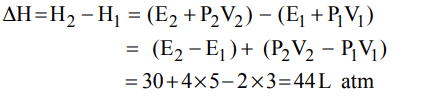
8. The enthalpy of vapourisation of water from the following
two equations is.
\[H_{2}\left(g\right)+\frac{1}{2}O_{2}\left(g\right)\rightarrow H_{2}O\left(l\right),\triangle\] H=-286kJ
\[H_{2}\left(g\right)+\frac{1}{2}O_{2}\left(g\right)\rightarrow H_{2}O\left(g\right),\triangle\] H=-245.5kJ
a) 6.02 kJ
b) 40.5 kJ
c) 62.3 kJ
d) 1.25 kJ
Explanation:
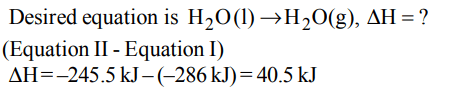
9. The variation of heat of reaction with temperature is given by:
a) Van’t Hoff equation
b) Clausius- Clapeyron equation
c) Nernst equation
d) Kirchoff’s equation
Explanation:
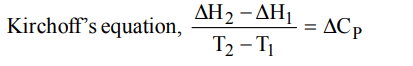
10. Kirchoff’s equation is :
a) \[K=Ae^{-E_{a}/RT}\]
b) \[\log_{}{\frac{K_{2}}{K_{1}}}=\frac{E_{a}}{2.303R}\left(\frac{T_{2}-T_{1}}{T_{1}T_{2}}\right)\]
c) \[E_{CeII}=\frac{2.303RT}{nF}\log_{}{\frac{C_{2}}{C_{1}}}\]
d) \[\frac{\triangle H_{2}-\triangle H_{1} }{T_{2}-T_{1}}=\triangle C_{P}\]
Explanation: \[\frac{\triangle H_{2}-\triangle H_{1} }{T_{2}-T_{1}}=\triangle C_{P}\]