1. The standard Gibb’s free energy change, \[\triangle G^{\circ}\] is related to equilibrium constant, \[K_{P}\] as
a) \[K_{P}=-RT ln\triangle G^{\circ}\]
b) \[K_{P}=\left[\frac{e}{RT}\right]^{\triangle G^{\circ}}\]
c) \[K_{P}=-\frac{{\triangle G}}{RT}\]
d) \[K_{P}=e^{-\triangle G^{\circ}/RT}\]
Explanation: \[\triangle\]G = - RT ln Kp
\[K_{P}=e^{-\triangle G^{\circ}/RT}\]
2. What is the free energy change. \[\triangle G\] when 1.0 mole of water at 100º C and 1 atm pressure is converted into steam at 100°C and 1 atm. pressure
a) 540 cal
b) –9800 cal
c) 9800 cal
d) 0 cal
Explanation: Condition of equilibrium, hence \[\triangle\]G = 0
3. According to third law of thermodynamics, which one of the following quantities for a perfectly crystalline solid is zero at absolute zero.
a) Entropy
b) Free energy
c) Internal energy
d) Enthalpy
Explanation: Statement of third law
4. The calorific value of fat is
a) less than that of carbohydrate and protein
b) less than that of protein but more than carbohydrate
c) less than that of carbohydrate but more than that of protein
d) more than that of carbohydrate and protein.
Explanation: Calorific value of fat > carbohydrate and protein; Butter 34, Sugar 16, Egg 14
5. Heat of neutralisation of strong acid against strong base is constant and is equal to
a) 13.7 kcal
b) 57 kJ
c) \[5.7 ×10^{4}J\]
d) All of the above
Explanation: All values are same
6. The heat of formation of the compound in the following reaction is
\[H_{2}\left(g\right)+CI_{2}\left(g\right)\rightarrow 2HCI\left(g\right)+ 44kcal\]
a) \[-44 kcalmol^{-1}\]
b) \[-22 kcalmol^{-1}\]
c) \[11 kcalmol^{-1}\]
d) \[-88 kcalmol^{-1}\]
Explanation: For one mole the value is - 22 kcal mol-1
7. Given that \[C+O_{2}\rightarrow CO_{2}\triangle H^{\circ}=-xkJ\]
\[2CO+O_{2}\rightarrow 2CO_{2}\triangle H^{\circ}=-ykJ\]
the enthalpy of formation of carbon monoxide will be
a) \[\frac{2x-y}{2}\]
b) \[\frac{y-2x}{2}\]
c) 2x - y
d) y = 2x.
Explanation:
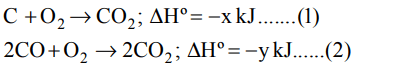
multiply equation (1) by 2 and substract equation (2) and divide final equation by 2
8. The enthalpy of neutralization of acetic acid and sodium hydroxide is –55.4 kJ. What is the enthalpy of ionization of acetic acid ?
a) –1.9 kJ
b) +1.9 kJ
c) +5.54 kJ
d) –5.54 kJ
Explanation: 57.3 – 55.4 = 1.9 kJ (57.3 kJ is heat of neutralisation of strong acid and strong base)
9. The neutralisation of a strong acid by a strong base liberates an amount of energy per mole of \[H^{+}\]
a) depends upon which acid and base are involved
b) depends upon the temperature at which the reaction takes place
c) depends upon which catalyst is used
d) is always the same
Explanation: It is infact the formation of one molecule of H2O always
(H+ + OH- \[\rightarrow\] H2O)
10. Equal volumes of methanoic acid and sodium hydroxide are mixed. If x is the heat of formation of water, then heat evolved on neutralization is
a) more than x
b) equal to x
c) less than x
d) twice x.
Explanation: Neutralisation of weak acid with strong base hence < x. Extra heat is utilised to effect the ionisation of weak acid.