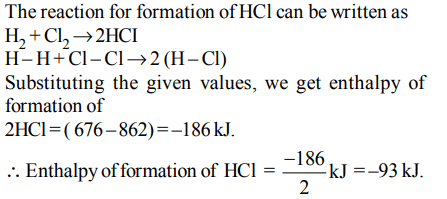
1. Bond dissociation enthalpy of \[H_{2}, Cl_{2} and HCl\] are 434 , 242 and 431 kJ \[mol^{-1}\] respectively. Enthalpy of formation of HCl is :
a) \[93 kJ mol^{-1}\]
b) \[– 245 kJ mol^{-1}\]
c) \[– 93 kJ mol^{-1}\]
d) \[245 kJ mol^{-1}\]
Explanation:
2. The values of \[\triangle H and \triangle S\] for the reaction, \[C\left(graphite\right)+CO_{2}\left(g\right)\rightarrow 2CO\left(g\right)\] are 170 kJ and \[170 JK^{-1}\] , respectively. This reaction will be spontaneous at
a) 910 K
b) 1110 K
c) 510 K
d) 710 K
Explanation:
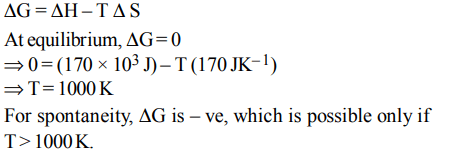
3. Standard entropies of \[X_{2},Y_{2}and XY_{3}\] are 60, 40 and \[50 JK^{-1}mol^{-1}\] respectively. For the reaction
\[\frac{1}{2}X_{2}+\frac{3}{2}Y_{2}\rightleftharpoons XY_{3},\triangle H=– 30 kJ\]
to be at equilibrium, the temperature should be:
a) 750 K
b) 1000 K
c) 1250 K
d) 500 K
Explanation:
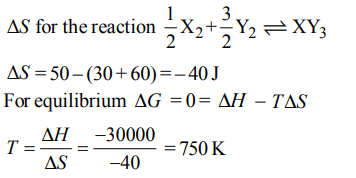
4. For vaporization of water at 1 atmospheric pressure, the values of \[\triangle H and \triangle S\] are \[40.63 k J mol^{-1} and 108.8 JK^{-1}mol^{-1}\] respectively. The temperature when Gibbs energy change
\[\left(\triangle G \right)\] for this transformation will be zero, is:
a) 293.4 K
b) 273.4 K
c) 393.4 K
d) 373.4 K.
Explanation:
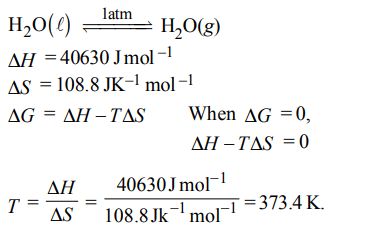
5. The following two reactions are known :
\[Fe_{2}O_{3}\left(s\right)+3CO\left(g\right)\rightarrow 2Fe\left(s\right)+3CO_{2}\left(g\right);\triangle H= –26.8 kJ\]
\[FeO\left(s\right)+CO\left(g\right)\rightarrow Fe\left(s\right)+CO_{2}\left(g\right);\triangle H= –16.5 kJ\]
The value of \[\triangle H\] for the following reaction
\[Fe_{2}O_{3}\left(s\right)+CO\left(g\right)\rightarrow 2FeO\left(s\right)+CO_{2}\left(g\right)\]
a) + 6.2 kJ
b) + 10.3 kJ
c) – 43.3 kJ
d) – 10.3 kJ
Explanation:
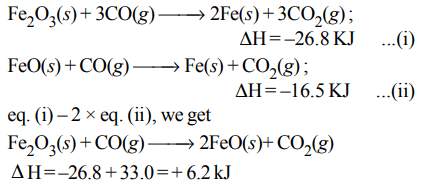
6. Three moles of an ideal gas expanded spontaneously into vacuum. The work done will be :
a) Zero
b) Infinite
c) 3 Joules
d) 9 Joules
Explanation: Ideal gas during spontaneous expansion into vacuum does not do any external work.
7. If the enthalpy change for the transition of liquid water to steam is \[30 kJ mol^{-1}\] at 27ºC, the entropy change for the process would be :
a) \[10 J mol^{-1}K^{-1}\]
b) \[1.0 J mol^{-1}K^{-1}\]
c) \[0.1 J mol^{-1}K^{-1}\]
d) \[100 J mol^{-1}K^{-1}\]
Explanation:
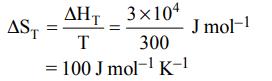
8. Enthalpy change for the reaction,\[4H\left(g\right)\rightarrow2H_{2}\left(g\right)\] is – 869.6 kJ.
The dissociation energy of H–H bond is :
a) – 434.8 kJ
b) – 869.6 kJ
c) + 434.8 kJ
d) + 217.4 kJ
Explanation:
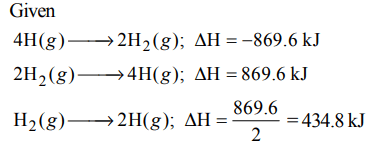
9. Consider the following processes :
\[1\diagup2 A\rightarrow B\] +150 kJ/mol
\[3B\rightarrow 2C+D\] –125 kJ/mol
\[E+A\rightarrow2D\] +350 kJ/mol
For \[B+D\rightarrow E+2C,\triangle H\] will be :
a) 525 kJ/mol
b) – 175 kJ/mol
c) – 325 kJ/mol
d) 325 kJ/mol
Explanation:

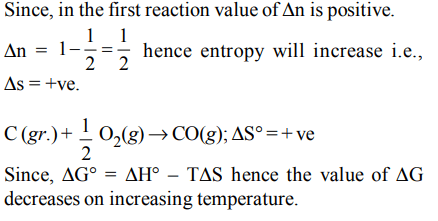
10. In which of the following reactions, standard entropy change (\[\triangle S^{\circ}\] is positive and standard Gibb’s energy change \[\triangle G^{\circ}\] decreases sharply with increase in temperature ?
a) \[C\left(graphite\right)+\frac{1}{2}O_{2}\left(g\right)\rightarrow CO\left(g\right)\]
b) \[CO\left(g\right)+\frac{1}{2}O_{2}\left(g\right)\rightarrow CO_{2}\left(g\right)\]
c) \[Mg\left(s\right)+\frac{1}{2}O_{2}\left(g\right)\rightarrow MgO\left(s\right)\]
d) \[\frac{1}{2}C\left(graphite\right)+\frac{1}{2}O_{2}\left(g\right)\rightarrow \frac{1}{2}CO_{2}\left(g\right)\]
Explanation: