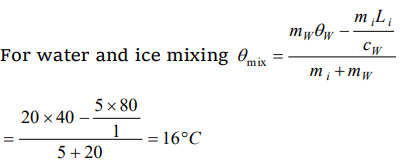1. A lead bullet of 10 g travelling at 300 m/s strikes against a block of wood and comes to rest. Assuming 50% of heat is absorbed by the bullet,
the increase in its temperature is (Specific heat of lead = 150J/kg, K)
a) 100°C
b) 125°C
c) 150°C
d) 200°C
Explanation:
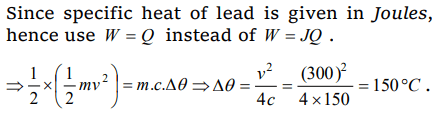
2. The temperature at which the vapour pressure of a liquid becomes equals to the external (atmospheric) pressure is its
a) Melting point
b) Sublimation point
c) Critical temperature
d) Boiling point
Explanation: At boiling point, vapour pressure becomes equal to the external pressure
3. When the pressure on water is increased the boiling temperature of water as compared to 100°C will be
a) Lower
b) The same
c) Higher
d) On the critical temperature
Explanation: When pressure increases boiling point also increases
4. Calorimeters are made of which of the following
a) Glass
b) Metal
c) Wood
d) Either (a) or (c)
Explanation: Calorimeters are made by conducting materials
5. Triple point of water is
a) 273.16°F
b) 273.16 K
c) 273.16°C
d) 273.16 R
Explanation: Triple point of water is 273.16 K
6. A liquid boils when its vapour pressure equals
a) The atmospheric pressure
b) Pressure of 76.0 cm column of mercury
c) The critical pressure
d) The dew point of the surroundings
Explanation: The atmospheric pressure
7.The amount of work, which can be obtained by supplying 200 cal of heat, is
a) 840 dyne
b) 840 W
c) 840 erg
d) 840 J
Explanation: W = JQ
W = 4.2 * 200 = 840 J
8. How many grams of a liquid of specific heat 0.2 at a temperature 40°C must be mixed with 100 gm of a liquid of specific heat of 0.5 at a temperature
20°C, so that the final temperature of the mixture becomes 32°C
a) 175 gm
b) 300 g
c) 295 gm
d) 375 g
Explanation:
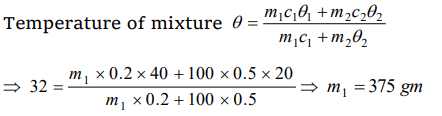
9. 1 g of a steam at 100°C melt how much ice at 0°C? (Latent heat of ice = 80 cal/gm and latent heat of steam = 540 cal/gm)
a) 1 gm
b) 2 gm
c) 4 gm
d) 8 gm
Explanation:
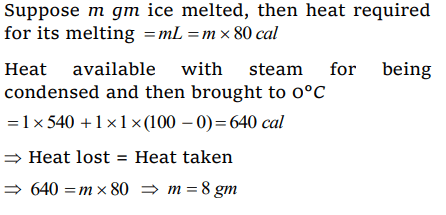
10. 5 g of ice at 0°C is dropped in a beaker containing 20 g of water at 40°C. The final temperature will
be
a) 32°C
b) 16°C
c) 8°C
d) 24°C
Explanation: