1. Hailstone at 0°C falls from a height of 1 km on an insulating surface converting whole of its kinetic
energy into heat. What part of it will melt \[\left(g=10m\diagup s^{2}\right)\]
a) \[\frac{1}{33}\]
b) \[\frac{1}{8}\]
c) \[\frac{1}{33}\times 10^{-4}\]
d) All of it will melt
Explanation: Suppose m' kg ice melts out of m kg then by using
W = JQ \[\Rightarrow\] mgh = J(m'L) . Hence fraction of ice melts
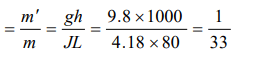
2. The SI unit of mechanical equivalent of heat is
a) \[Joule \times Calorie\]
b) Joule/Calorie
c) \[Calorie \times Erg\]
d) Erg/Calorie
Explanation:
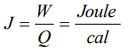
3. Of two masses of 5 kg each falling from height of 10 m, by which 2kg water is stirred. The rise in temperature of water will be
a) 2.6°C
b) 1.2°C
c) 0.32°C
d) 0.12°C
Explanation:
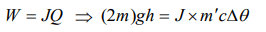
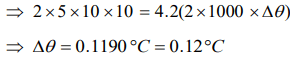
4. A lead ball moving with a velocity V strikes a wall and stops. If 50% of its energy is converted into heat, then what will be the increase in temperature (Specific heat of lead is S)
a) \[\frac{2V^{2}}{JS}\]
b) \[\frac{V^{2}}{4JS}\]
c) \[\frac{V^{2}}{J}\]
d) \[\frac{V^{2}S}{2J}\]
Explanation:
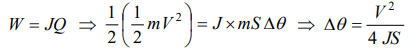
5. The mechanical equivalent of heat J is
a) A constant
b) A physical quantity
c) A conversion factor
d) None of the above
Explanation: ‘J’ is a conversion
6. Water falls from a height of 210m. Assuming whole of energy due to fall is converted into heat the rise in temperature of water would be (J = 4.3
Joule/cal)
a) 42°C
b) 49°C
c) 0.49°C
d) 4.9°C
Explanation:
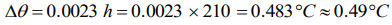
7. A block of mass 100 gm slides on a rough horizontal surface. If the speed of the block decreases from 10 m/s to 5 m/s, the thermal
energy developed in the process is
a) 3.75 J
b) 37.5 J
c) 0.375 J
d) 0.75 J
Explanation:
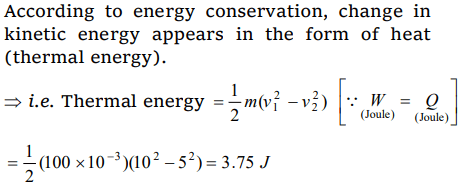
8. 4200 J of work is required for
a) Increasing the temperature of 10 gm of water through 10°C
b) Increasing the temperature of 100 gm of water through 10°C
c) Increasing the temperature of 1 kg of water through 10°C
d) Increasing the temperature of 10 kg of water through 10°C
Explanation: Work done to raise the temperature of 100 gm water through 10°C is W = JQ = 4.2 * (100 * 10-3 * 1000 * 10) = 4200 J
9. At 100°C, the substance that causes the most severe burn, is
a) Oil
b) Steam
c) Water
d) Hot air
Explanation: Among all the option, latent heat of steam is highest
10. In a water-fall the water falls from a height of 100 m. If the entire K.E. of water is converted into
heat, the rise in temperature of water will be
a) 0.23°C
b) 0.46°C
c) 2.3°C
d) 0.023°C
Explanation: \[\triangle \theta\] = 0.0023 h = 0.0023 * 100 = 0.23°C