1. A certain gas takes three times as long to effuse out as helium. Its molecular mass will be :
a) 27 u
b) 36 u
c) 64 u
d) 9 u
Discussion
Explanation:
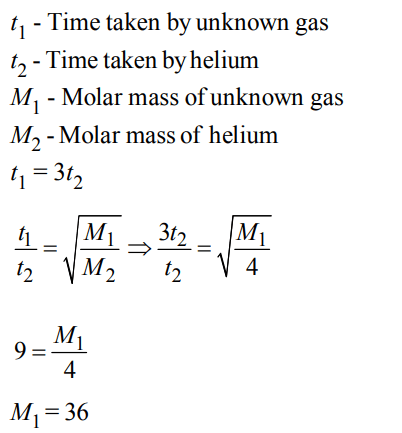
2. Maximum deviation from ideal gas is expected from :
a) \[N_{2}\left(g\right)\]
b) \[CH_{4}\left(g\right)\]
c) \[NH_{3}\left(g\right)\]
d) \[H_{2}\left(g\right)\]
Discussion
Explanation: Higher the critical temperature more easily will be the gas liquify. Now since most easily liquifiable gas show larger deviation, NH3 will show maximum deviation from ideal behaviour.
3. For an ideal gas, number of moles per litre in terms of its presure P, gas constant R and temperature T is
a) PT/R
b) PRT
c) P/RT
d) RT/P
Discussion
Explanation: PV = nRT (number of moles = n/V)
n/V = P/RT
4. Value of gas constant R is
a) 0.082 litre atm
b) \[0.987 cal mol^{-}K^{-1}\]
c) \[8.3 J mol^{-}K^{-1}\]
d) \[83 erg mol^{-}K^{-1}\]
Discussion
Explanation: \[8.3 J mol^{-}K^{-1}\]
5. Kinetic theory of gases proves
a) only Boyle’s law
b) only Charles’ law
c) only Avogadro’s law
d) All of these
Discussion
Explanation: All of these
6. The heat required to raise the temperature of body by 1 K is called
a) specific heat
b) thermal capacity
c) water equivalent
d) none of these
Discussion
Explanation: The heat rquired to raise the temperature of body by 1K is called thermal capacity or heat capacity.
7. What volume of hydrogen gas, at 273 K and 1 atm pressure will be consumed in obtaining 21.6 g of elemental boron (atomic mass = 10.8) from the reduction of boron trichloride by hydrogen ?
a) 67.2 L
b) 44.8 L
c) 22.4 L
d) 89.6 L
Discussion
Explanation:
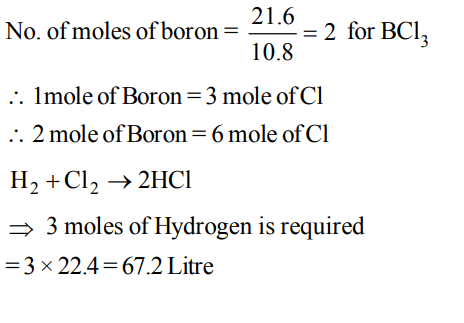
8. According to the kinetic theory of gases, in an ideal gas, between two successive collisions a gas molecule travels
a) in a wavy path
b) in a straight line path
c) with an accelerated velocity
d) in a circular path
Discussion
Explanation: According to kinetic theory the gas molecules travel in a straight line path but show zig-zag motion due to collisions.
9. As the temperature is raised from 20ºC to 40ºC, the average kinetic energy of neon atoms changes by a factor of which of the following ?
a) \[313\diagup293\]
b) \[\sqrt{\left(313\diagup293\right)}\]
c) \[1\diagup2\]
d) 2
Discussion
Explanation:
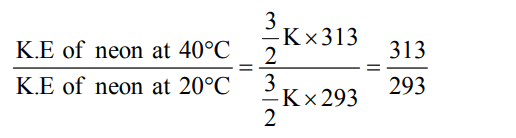
10. In van der Waals equation of state of the gas law, the constant
‘b’ is a measure of
a) volume occupied by the molecules
b) intermolecular attraction
c) intermolecular repulsions
d) intermolecular collisions per unit volume
Discussion
Explanation: In van der waal's equation ‘b’ is for volume correction