1. Boyle’s law, according to kinetic equation can be expressed as
a) PV = KT
b) PV = RT
c) \[PV=\frac{3}{2}kT\]
d) \[PV=\frac{2}{3}kT\]
Discussion
Explanation:
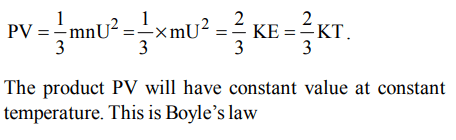
2. The ratio between the root mean square velocity of \[H_{2}\] at 50 K and that of \[O_{2}\] at 800 K is
a) 4
b) 2
c) 1
d) 1/4
Discussion
Explanation:
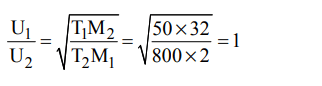
3.The r.m.s velocity of hydrogen is \[\sqrt{7}\] times the r.m.s velocity of nitrogen. If T is the temperature of the gas , than
a) \[T\left(H_{2}\right)=T\left(N_{2}\right)\]
b) \[T\left(H_{2}\right)>T\left(N_{2}\right)\]
c) \[T\left(H_{2}\right)<T\left(N_{2}\right)\]
d) \[T\left(H_{2}\right)=\sqrt{7}\left(N_{2}\right)\]
Discussion
Explanation:
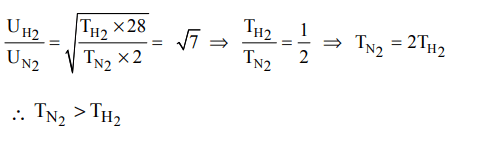
4. The r.m.s velocity of \[CO_{2}\] at temperature T (in kelvin) is \[x cms^{-1}\] . At what temperature (in kelvin) the r.m.s. velocity of nitrous oxide would be \[4x cms^{-1}\] ?
a) 16 T
b) 2 T
c) 4 T
d) 32 T
Discussion
Explanation:
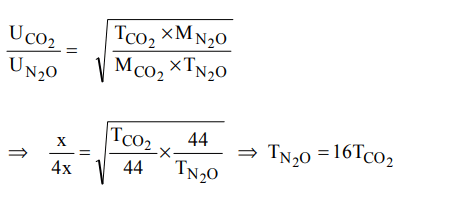
5. Which of the following has maximum root mean square velocity at the same temperature?
a) \[SO_{2}\]
b) \[CO_{2}\]
c) \[O_{2}\]
d) \[H_{2}\]
Discussion
Explanation: \[U \propto \sqrt{\frac{1}{M}} \] hence hydrogen (molecular weight being the lowest) has the maximum root mean square velocity
6. Density ratio of \[O_{2} and H_{2}\] is 16:1. The ratio of their r.m.s. velocities will be
a) 4 : 1
b) 1 : 16
c) 1 : 4
d) 16 : 1
Discussion
Explanation: \[\frac{U_{1}}{U_{2}}= \sqrt{\frac{d_{2}}{d_{1}}} = \sqrt{\frac{1}{16}} = 1:4\]
7. At what temperature the RMS velocity of \[SO_{2} \] be same as that of \[O_{2} \] at 303 K ?
a) 273 K
b) 606 K
c) 303 K
d) 403 K
Discussion
Explanation: Apply \[\frac{U_{1}}{U_{2}} = \sqrt{\frac{T_{1}M_{2}}{T_{2}M_{1}}} \]
8. The temperature of an ideal gas is reduced from 927ºC to 27ºC. the r.m.s. velocity of the molecules becomes
a) double the inital value
b) half of the initial value
c) four times the initial value
d) ten times the initial value
Discussion
Explanation:
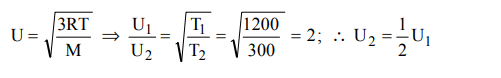
9. If the average velocity of \[N_{2} \] molecues is 0.3 m/s at 27ºC, then the velocity of 0.6 m/s will take place at
a) 273 K
b) 927 K
c) 1000 K
d) 1200 K
Discussion
Explanation: 1200 K
10. Gas deviates from ideal gas nature because molecules
a) are colouress
b) attaract each other
c) contain covalent bond
d) show Brownian movement
Discussion
Explanation: Due to intermolecular interactions appreciable at high P and low T, the ideal gas deviates from ideal behaviour.