1. According to VSEPR theory, the most probable shape of the molecule having 4 electron pairs in the outershell of the central atom is
a) linear
b) tetrahedral
c) hexahedral
d) octahedral
Explanation: Tetrahedral
2. The geometry of \[CIO_3^-\] ion according to Valence Shell Electron Pair Repulsion (VSEPR) theory will be
a) planar triangular
b) pyramidal
c) tetrahedral
d) square planar
Explanation: Hybridisation is sp3 and shape pyramidal
3. Among the following ions, the \[p\pi-d\pi\] overlap could be present in
a) \[NO_3^-\]
b) \[PO_4^{3-}\]
c) \[CO_3^{2-}\]
d) \[NO_2^-\]
Explanation: Hybridisation in \[PO_4^{3-}\] = ½ [5 + 0 + 3 –0] = 4 sp3 . In \[\pi\] bonding only d orbital of P, p orbital of O can be involved. Since hybrid atomic orbitals do not form \[\pi\] bond.
4. In \[NO_3^-\] ion, number of bond pairs and lone pairs of electrons on nitrogen atom are
a) 2, 2
b) 3,1
c) 1,3
d) 4,0
Explanation:
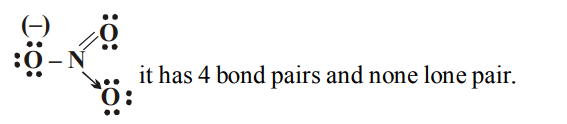
5. In \[OF_{2}\], number of bond pairs and lone pairs of electrons are respectively
a) 2,6
b) 2,8
c) 2,10
d) 2,9
Explanation:
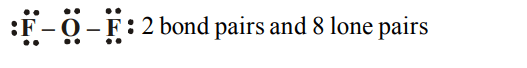
6. The compound containing co-ordinate bond is
a) \[H_{2}SO_{4}\]
b) \[O_{3}\]
c) \[SO_{3}\]
d) All of these
Explanation:
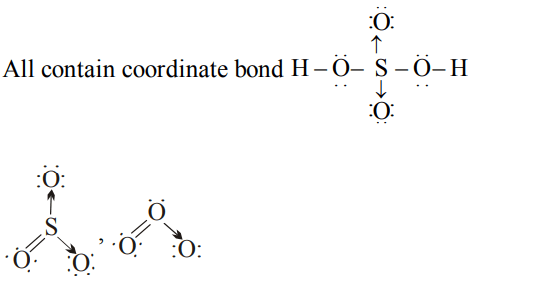
7. Sulphuric acid provides a simple example of
a) co-ordinate bonds
b) non-covalent compound
c) covalent ion
d) non - covalent ion
Explanation: Coordinate bond
8. Which of the following does not contain coordinate bond ?
a) \[BH_4^-\]
b) \[NH_4^+\]
c) \[CO_3^{2-}\]
d) \[H_{3}O^{+}\]
Explanation:
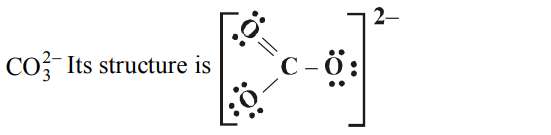
9. The maximum number of water molecules that one water molecule can hold through hydrogen bonding is
a) 2
b) 4
c) 6
d) 8
Explanation: It is 4 See H - bonding
10. \[NH_{3}\] has abnormally high boiling point because it has
a) alkaline nature
b) distorted shape
c) \[sp^{3}\] - Hybridization
d) hydrogen bonding
Explanation: NH3 forms intermolecular H - bonding.