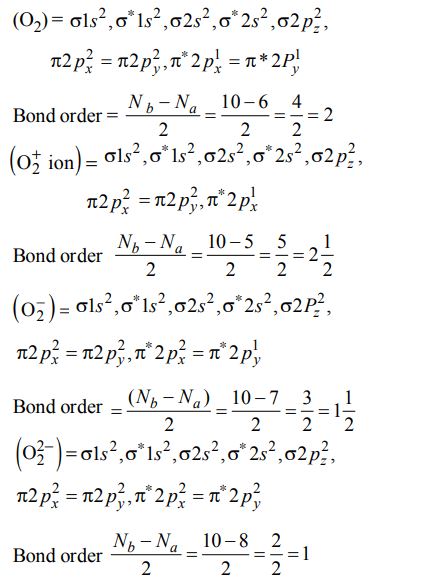1. In which one of the following species the central atom has the type of hybridization which is not the same as that present in the other three?
a) \[SF_{4}\]
b) \[I_3^-\]
c) \[SbCl_5^{2-}\]
d) \[PCI_{5}\]
Explanation: For \[SbCl_5^{2-}\] , H = \[\frac{5+5+2}{2}\] = 6 means sp3d2 hybridization
\[I_3^-\], \[SF_{4}\], and \[PCI_{5}\] sp3d hybridization
2. Some of the properties of the two species, \[NO_3^- and H_{3}O^{+}\] are described below. Which one of them is correct?
a) Similar in hybridization for the central atom with different
structures.
b) Dissimilar in hybridization for the central atom with
different structures
c) Isostructural with same hybridization for the central
atom
d) Isostructural with different hybridization for the central
atom
Explanation: In \[NO_3^-\], nitrogen have sp2 hybridisation, thus planar in shape. In \[H_{3}O^{+}\], oxygen is in sp3 hybridisation, thus tetrahedral geometry is expected but due to presence of one lp of electrons on central oxygen atom it is pyramidal in shape
3. In which of the following molecules the central atom does not have \[sp^{3}\] hybridization?
a) \[NH_4^+\]
b) \[CH_{4}\]
c) \[SF_{4}\]
d) \[BF_4^-\]
Explanation: \[NH_4^+\] : sp3 hybridisation
\[CH_{4}\] : sp3 hybridisation
\[SF_{4}\] : sp3d hybridisation
\[BF_4^-\] : sp3 hybridisation
4. Which one of the following species does not exist under normal conditions?
a) \[Be_2^+\]
b) \[Be_{2}\]
c) \[B_{2}\]
d) \[Li_{2}\]
Explanation: Bond order of \[Be_{2}\] = 0, hence \[Be_{2}\] cannot exist
5. Considering the state of hybridization of carbon atoms, find out the molecule among the following which is linear ?
a) \[CH_{3}-CH=CH-CH_{3}\]
b) \[CH_{3}-C=C-CH_{3}\]
c) \[CH_{2}=CH-CH_{2}-C=CH\]
d) \[CH_{3}-CH_{2}-CH_{2}-CH_{3}\]
Explanation:
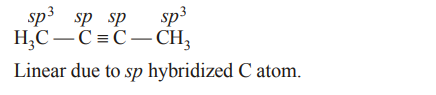
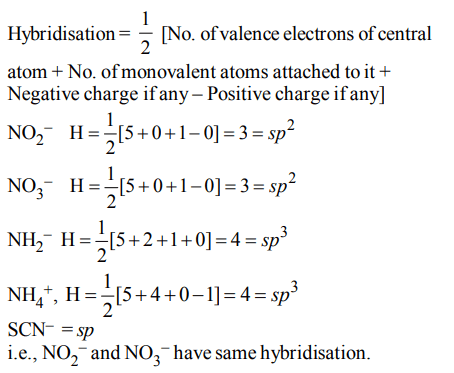
6. Which of the two ions from the list given below have the geometry that is explained by the same hybridization of
orbitals, \[NO_2^-,NO_3^-,NH_2^-,NH_4^+.SCN^{-}\] ?
a) \[NO_2^- and NO_3^- \]
b) \[NH_2^- and NO_3^- \]
c) \[SCN^{-} and NH_2^- \]
d) \[NO_2^- and NH_2^-\]
Explanation:
7. Which of the following has the minimum bond length ?
a) \[O_2^+\]
b) \[O_2^-\]
c) \[O_2^{2-}\]
d) \[O_{2}\]
Explanation:
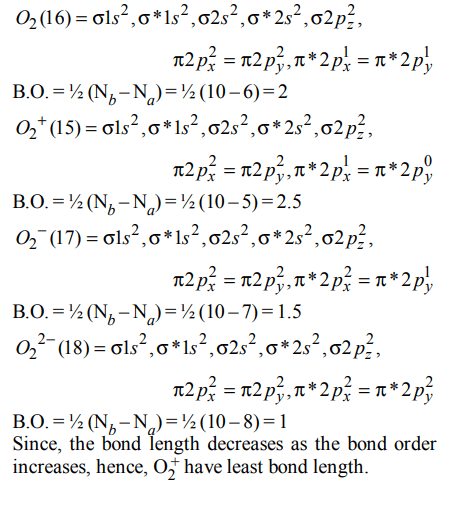
8. The pairs of species of oxygen and their magnetic behaviours are noted below. Which of the following presents
the correct description ?
a) \[O_2^-,O_2^{2-}\] – Both diamagnetic
b) \[O_2^-,O_2^{2+}\] – Both paramagnetic
c) \[O_2^+,O_{2}\] – Both paramagnetic
d) None of the above
Explanation:
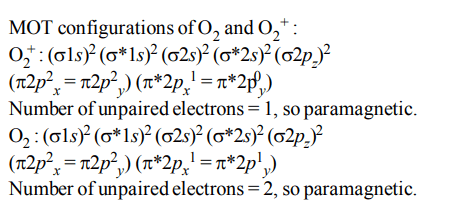
9.Which one of the following pairs is isostructural (i.e., having the same shape and hybridization) ?
a) \[\left[BCI_{3} and BrCI_3^-\right]\]
b) \[\left[NH_{3} and NO_3^-\right]\]
c) \[NF_{3} and BF_{3}\]
d) \[\left[BF_4^- and NH_4^+\right]\]
Explanation: \[BF_4^-\] hybridisation sp3 , tetrahedral structure
\[NH_4^+\] hybridisation sp3 , tetrahedral structure
10. Bond order of 1.5 is shown by :
a) \[O_2^+\]
b) \[O_2^-\]
c) \[O_2^{2-}\]
d) \[O_{2}\]
Explanation: