1. Which of the following pairs have identical bond order ?
a) \[N_{2},O_2^{2+}\]
b) \[N_{2},O_2^{-1}\]
c) \[N_2^-,O_{2}\]
d) \[O^{2+},N_{2}\]
Explanation: Bond order in \[N_{2},O_2^{2+}\] is 3
2. Which one of the following should be most stable?
a) \[H_2^+\]
b) \[H^{+}\]
c) H
d) \[H^{-}\]
Explanation: \[H^{+}\]
3. The number of antibonding electron pairs in \[O_2^{2-}\] molecularion on the basis of molecular orbital theory are (at. no. O = 8)
a) 2
b) 3
c) 4
d) 5
Explanation:
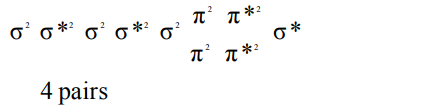
4. Which of the following is not paramagnetic ?
a) \[H_2^+\]
b) CO
c) \[O_2^-\]
d) NO
Explanation: CO is diamagnetic while others are paramagnetic
5. Which of the following molecular species has unpaired electron (s) ?
a) \[N_{2}\]
b) \[F_{2}\]
c) \[O_2^-\]
d) \[O_2^{2-}\]
Explanation: \[O_2^-\]
6. Which of the following has least covalent P–H bond?
a) \[PH_{3}\]
b) \[P_{2}H_{6}\]
c) \[P_{2}H_{5}\]
d) \[PH_6^+\]
Explanation: In \[PH_6^+\], P – H bond will have ionic character due to electron attracting tendency of P carrying +ve charge and least covalent.
7. Which contains both polar and non - polar bonds?
a) \[NH_{4}Cl\]
b) HCN
c) \[H_{2}O_{2}\]
d) \[CH_{4}\]
Explanation: \[H_{2}O_{2}\]
8. Which of the following has least polarity in bond?
a) H – F
b) H – Cl
c) H – O
d) H – S
Explanation: The electronegativity of S is least among others hence H -S bond is least polar in nature.
9. Which of the following substances has the least ionic character ?
a) \[FeCI_{2}\]
b) \[ZnCI_{2}\]
c) \[CdCI_{2}\]
d) \[MgCI_{2}\]
Explanation: Polarizing power of Zn2+ is more than others, hence ZnCl2 is least ionic in nature.
10. The correct order of increasing ionic character is
a) \[BeCI_{2}<MgCI_{2}<CaCI_{2}<BaCI_{2}\]
b) \[BeCI_{2}<MgCI_{2}<BaCI_{2}<CaCI_{2}\]
c) \[BeCI_{2}<BaCI_{2}<MgCI_{2}<CaCI_{2}\]
d) \[BaCI_{2}<CaCI_{2}<MgCI_{2}<BeCI_{2}\]
Explanation: Electropositive character and size increases down the group, the ionic character increases.