1. When zeolite (hydrated sodium aluminium silicate) is treated with hard water the sodium ions are exchanged with
a) \[H^{+}ions\]
b) \[Ca^{2+}ions\]
c) \[SO_4^{2-} ions\]
d) \[OH^{-}ions\]
Explanation:
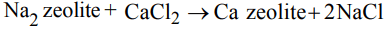
2. The species that does not contain peroxide ions
a) \[PbO_{2}\]
b) \[H_{2}O_{2}\]
c) \[SrO_{2}\]
d) \[BaO_{2}\]
Explanation:
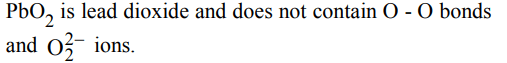
3. Hydrogen peroxide does not
a) liberate iodide from KI
b) turn titanium salt yellow
c) gives silver peroxide with moist silver oxide
d) turn mixture of aniline, \[KClO_{3}\] and dil. \[H_{2}SO_{4}\] violet
Explanation: No such reaction is known (c).
4. 30 volume hydrogen peroxide means
a) 30% of \[H_{2}O_{2}\] solution
b) \[30cm^{3}\] solution contains 1g of \[H_{2}O_{2}\]
c) \[1cm^{3}\] of solution liberates 30 cm3 of O2 at STP
d) \[30cm^{3}\] of solution contains 1 mole of \[H_{2}O_{2}\]
Explanation: 30 vol of H2O2 means one volume of H2O2 on decomposition will give 30 volume of oxygen
5. The molarity of a 100 ml solution containing 5.1 g of hydrogen peroxide is
a) 0.15 M
b) 1.5 M
c) 3.0 M
d) 50.0 M
Explanation:
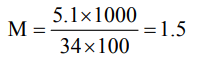
6. The oxidation states of most electronegative element in the products of reaction \[BaO_{2}\] with dil. \[H_{2}SO_{4}\] are
a) 0 and – 1
b) –1 and – 2
c) – 2 and 0
d) – 2 and + 1
Explanation:
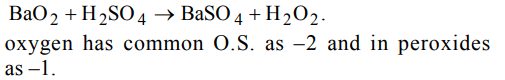
7. Permanent hardness of water can be removed by adding Calgon \[\left(NaPO_{3}\right)_{n}\] . This is an example of
a) adsorption
b) exchange of ion
c) precipitation
d) none of these
Explanation: There is exchange of ions
8. Water contracts on heating
a) to 100°C
b) from 0°C to 4°C
c) to 273 K
d) from 10°C to 20°C
Explanation:

9. 1000g aqueous solution of \[CaCO_{3}\] contains 10g of calcium carbonate . Hardness of solution is
a) 10 ppm
b) 100 ppm
c) 1000 ppm
d) 10000 ppm
Explanation:
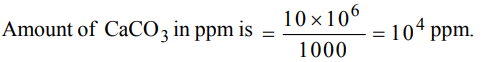
10. Which statement is wrong?
a) Ordinary hydrogen is an equilibrium mixture of ortho and para hydrogen
b) In ortho hydrogen spin of two nuclei is in same direction
c) Ortho and para forms do not resemble in their chemical properties
d) In para hydrogen spin of two nuclei is in opposite direction
Explanation: Ortho and para forms of hydrogen resemble in their chemical properties.